Most common problems are false positives, inhibition of PCR and sample switching

One of the purposes of inter-laboratory performance testing, also known as a ring test, is to assure the clinicians, clients or regulatory officials that the results provided are accurate and specific. Another purpose is to determine if the test methods in use are reliable and reproducible.
As a World Organisation for Animal Health (OIE) reference laboratory and United States Department of Agriculture Animal and Plant Health Inspection Service-approved laboratory for crustacean pathogens, the University of Arizona’s Aquaculture Pathology Laboratory has taken on the role of providing training and assistance to other shrimp diagnostic laboratories for the detection of shrimp pathogens through the use of polymerase chain reaction (PCR) testing. The lab has been routinely implementing ring tests since 2005.
To date, laboratories from 10 countries – Brazil, Brunei, Ecuador, Madagascar, Mexico, Panama, the Philippines, Saudi Arabia, United States and Vietnam – have participated in these ring tests (Fig. 1). The majority of these labs correctly diagnosed the unknown samples, which indicated high proficiency.
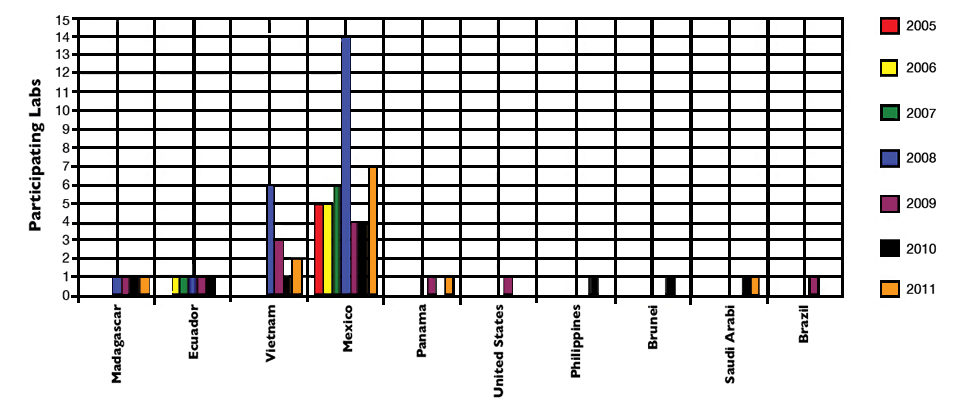
Participation
Participation in the ring testing is completely voluntary. There are no prescribed methods, and each participating laboratory employs the same PCR procedures it routinely uses in the analysis of clinical samples.
Each laboratory is provided with a panel of 10 coded tissue samples fixed in 95 percent ethanol. Five of these samples are for DNA extraction, and the remaining five are for RNA extraction. The following pathogens have been present in the panels: white spot syndrome virus, infectious hypodermal and hematopoietic necrosis virus, necrotizing hepatopancreatitis bacteria, Taura syndrome virus, yellow head virus and infectious myonecrosis virus. There is also tissue free of specific pathogens. Only one of these pathogens is present in each sample.
Each participating laboratory tests only for those pathogens they are set up to analyze on a routine basis. However, if a laboratory is ready and interested, it may chose to test for all the pathogens.
A standard report format is provided, and the laboratories are given a maximum of seven working days to analyze the samples. For the reports to be considered complete, the primer sets or commercial kits, extraction methods, PCR conditions and gel photographs must be included in the participating labs’ reports. Some laboratories employ real-time PCR and include their chromatograms in the results section of the report.
Ring reporting, funding
The results from each laboratory are evaluated and summarized by the University of Arizona Aquaculture Pathology Laboratory, and compiled into a final report distributed to all participants. In this summary report, the laboratories are referenced by code letters to maintain confidentiality. Since the purpose of the ring test is not only to determine proficiency, but to help improve performance, feedback is also provided to those laboratories experiencing problems with the analysis.
Commercial PCR kits are the most popular method of detection, followed by kits of regional production/distribution. Methods recommended by the World Organisation for Animal Health and methods published in specialized publications are also employed. The shortest turnaround time has been two days and the longest 13 days.
The ring test service is funded in great part by a participation fee paid by the laboratories. This fee covers the cost of producing infected tissues specifically for the test according to international standards and also pays for the time to evaluate results, troubleshoot and provide feedback. The ring test is offered twice a year, usually during the months of February and August. Most of the laboratories participate on a yearly basis, but some do request the service twice a year.
Common problems
The most common problems encountered are false positives related to contamination, inhibition of PCR (false negatives) and sample switching. Problems of low sensitivity, which also can lead to false negative results, are uncommon and usually associated with multiplex procedures/kits.
Perspectives
The benefits of the ring test are evolving and, for some laboratories, have become more than a self-evaluation exercise. In November 2010, a laboratory in Ciudad Obregón, Sonora, Mexico, which for years has shown high performance in these ring tests, obtained accreditation by the Mexican Accreditation Entity (EMA). Participation and performance in the ring test was part of the criteria applied by the EMA to grant this accreditation.
(Editor’s Note: This article was originally published in the May/June 2011 print edition of the Global Aquaculture Advocate.)
Authors
-
Carlos R. Pantoja, Ph.D.
University of Arizona
Aquaculture Pathology Laboratory
11117 East Lowell Street
Building 90, Room 106
Tucson, Arizona 85721 USA -
Solangel A. Navarro
University of Arizona
Aquaculture Pathology Laboratory
11117 East Lowell Street
Building 90, Room 106
Tucson, Arizona 85721 USA -

Donald V. Lightner, Ph.D.
University of Arizona
Aquaculture Pathology Laboratory
11117 East Lowell Street
Building 90, Room 106
Tucson, Arizona 85721 USA
Tagged With
Related Posts

Health & Welfare
A comprehensive look at the Proficiency Test for farmed shrimp
The University of Arizona Aquaculture Pathology Laboratory has carried out the Proficiency Test (PT) since 2005, with 300-plus diagnostic laboratories participating while improving their capabilities in the diagnosis of several shrimp pathogens.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Health & Welfare
Acclimating shrimp postlarvae before pond stocking
Shrimp postlarvae acclimation before stocking into the various growout systems (ponds, raceways, tanks) is a critical – and often overlooked, sometimes taken for granted – step in the shrimp culture process. Various water quality parameters should be changed slowly so that the young shrimp have the time to gradually adapt to the new conditions.

Health & Welfare
Big shoes to fill: Dhar takes reins at shrimp pathology laboratory
Arun Dhar, Ph.D. will attempt to fill the “big shoes” of Dr. Donald Lightner at the University of Arizona’s Aquaculture Pathology Laboratory, where the shrimp disease EMS was diagnosed.


