Author: A greater effort must be made to prepare lists of acceptable chemicals and recommendations for the use of these chemicals

Shrimp farmers in Asia and in the Americas have used a wide array of chemical and biological agents in attempts to control White Spot Syndrome Virus (WSSV) disease. Some of these substances are thought to kill the WSS virus or its potential carriers.
Other substances are claimed to stimulate the immune system of shrimp and help them resist the disease. Substances that improve environmental conditions in ponds – and thereby reduce stress – also are thought to enhance the resistance of shrimp to disease.
The agents used in these attempts may be applied to pond soil and water before shrimp are stocked, applied to the water during the crop, or added to the feed. Although some products may be beneficial when applied to aquaculture systems at low concentrations, they may be toxic to shrimp at higher concentrations, cause environmental problems in ponds or surrounding ecosystems, accumulate in shrimp tissue and present a food safety problem, or be hazardous to workers in the shrimp industry.
In this article, we will comment on the environmental, worker, and food safety status of these substances. This information was compiled from recent literature and the Internet site of the U.S. Environmental Protection Agency (EPA, http://www.epa.gov).
Insecticides
Three chemicals used in the effort against WSSV are insecticides; i.e., Sevin, Dipterex and Neem products. Diesel fuel also will be considered an insecticide because it is used to kill aquatic insects.
• Sevin, dipterex
According to EPA, Sevin is a moderately toxic carbamate insecticide. It can produce adverse effects in humans by skin contact, inhalation, or ingestion. However, evidence indicates that carbaryl, the main active ingredient in Sevin, is unlikely to be carcinogenic. Carbaryl inhibits cholinesterase, an enzyme necessary for normal nervous system functions in animals. Carbaryl can enter animals through ingestion or direct absorption from dermal contact. Some accumulation of carbaryl can occur in catfish, crawfish and snails, as well as in algae and duckweed. The residue level in fish may be 100X or more than the concentration of carbaryl in water. In general, carbaryl is metabolized and degraded rapidly and should not pose a significant bioaccumulation risk in alkaline waters of shrimp ponds. In surface water, carbaryl is broken down by bacteria and through hydrolysis, but volatilization from water is very slow. Carbaryl has a half-life of about 10 days at neutral pH, but its residence time increases greatly with increasing acidity.

Dipterex (Crustabay or Synterex) is an organophosphate and is classified by EPA as moderately toxic. The main active ingredient, trichlorfon, is moderately toxic by ingestion or dermal absorption. Skin sensitivity and allergies can result in humans from dermal exposure. Repeated or prolonged exposure to organophosphates may result in the same effects as acute exposure. Trichlorfon and other organophosphate compounds decrease the activity of the cholinesterase enzyme, and they are highly toxic to most aquatic species. EPA reports 96-hr LC50 values of 0.18 milligrams per liter in Daphnia, 0.01milligrams per liter in stoneflies, 7.8 milligrams per liter in crayfish, 1.4 milligrams per liter in rainbow trout, and 0.88 milligrams per liter in channel catfish. Toxicity in the field differs with species and can be affected by many factors including temperature, pH, and water hardness. Toxicity usually increases with higher temperature and higher pH. Studies did not show a potential for trichlorfon to accumulate in fish, and it degrades rapidly in alkaline water of pH 8.5 or higher. About 99 percent of applied trichlorfon is broken down within 2 hours after application in alkaline water, but it is stable for longer periods at lower pH. The major breakdown product of trichlorfon is dichlorvos (DDVP), also an insectcide. Dichlorvos persists at detectable levels for 526 days in water at 20 degrees-C.
Sevin and Dipterex should be handled with care. As with all cholinesterase inhibitors, acute exposure to high concentrations or chronic exposure to lesser concentrations can lead to a decline in blood cholinesterase levels, sickness, and even death. Workers should wear protective clothing and avoid inhalation of the vapor from these compounds. The antidote for poisoning by a cholinesterase inhibitor is atropine sulfate, and this substance should be available in case of an emergency. Spills of Sevin or Dipterex can be highly toxic in natural aquatic ecosystems around shrimp farms.
• Neem Extracts
Natural insecticides derived from products of the Neem tree (Azadirachta indica or Antelaea azadiracta) are also used occasionally. They are active against nematodes, fungi, and ostracods (Crustacea) at level of 10 ppm Neem Seed Kernel Extract (NSKE) (Schmutterer and Ascher 1987). There is no information concerning persistence of Neem extracts in aquatic environments; little is known about their toxicity or other effects on humans.
• Diesel Fuel
Production ponds often contain a large number of water boatman (Corixidae) and PCR analyses have shown that they are potential carriers of the WSS virus. Larvae of these aquatic insects concentrate at the air-water interface to breathe. Farmers try to control their numbers by applying diesel fuel to pond surfaces to prevent larvae from breathing. It is doubtful sufficient coverage of water surfaces by diesel can be achieved to control the larvae. In addition, it has been shown that fish and other aquatic animals exposed to petroleum products may develop off-flavors variously described as “oily,” “diesel fuel,” “petroleum,” or “kerosene,” and be rejected in the market.
Bactericides
Bactericides used in attempts to control WSSV include chlorine compounds, formaldehyde, providone-iodine, quaternary ammonia compounds, furazolidone, malachite green, calcium hydroxide and plant extracts.
Chlorine, associated compounds
The most common chlorine compounds for disinfection are chlorine gas (Cl2), calcium hypochlorite [Ca(OCl)2] and sodium hypochlorite (NaOCl). When chlorine gas is added to water, it hydrolyzes to form hypochlorous acid (HOCl) and hydrochloric acid (HCl):
Cl2 + H2O = HOCl + H+ + Cl–
Hydrochloric acid is completely ionized, and depending upon pH and temperature, hypochlorous acid ionizes to hydrogen ion (H+) and hypochlorite ion (OCl-):
HOCl = H+ + OCl–
Above pH 2, there will be essentially no Cl2 in water. The ionization of HOCl reaches 50 percent at pH 7.4, and at higher pH values, there will be more OCl- than HOCl. When sodium or calcium hypochlorite are added to water they also form HOCl and OCl- in relationship to pH and temperature. Hypochlorous acid and hypochlorite are called free chlorine residuals. The relative distribution of these two species is very important because the disinfecting efficiency of HOCl is 40 to 80 times that of OCl- (White 1992).
Chlorine readily oxidizes substances such as Fe2+, Mn2+, H2S and organic matter, and is reduced to nontoxic chloride ion. Once oxidation is complete, chlorine reacts with ammonia to form chloramines. After ammonia and other reduced substances have reacted with chlorine, continued addition of chlorine will result in a direct increase in free chlorine residuals (HOCl and OCl-). The main reason for adding enough chlorine to obtain a free chlorine residual is to ensure disinfection (White 1992). The amount of chlorine that must be added to reach a desired level of residual is called the chlorine demand.
Chlorination is a commonly used method for destruction of pathogenic and other harmful organisms in drinking water. In disinfection, enough chlorine is added to meet the chlorine demand and provide free chlorine residuals. Some organic constituents of water react with chlorine residuals to form toxic compounds such as trihalomethanes, dioxins, and polychlorinated biphenyls (PCBs). These compounds are suspected carcinogens (White 1992) and they may contaminate food. Dioxins and PCBs are thermally stable molecules that resist oxidation and hydrolysis and persist in the environment. EPA mentions that both dioxin and PCB molecules rarely cause acute toxicity in humans, but they have marked effects after repetitive or chronic exposures. The major threat associated with their presence in shrimp is the increasing concerns in international markets over contaminated food. The implementation of more stringent regulations for food importation will ultimately lead to the rejection of shrimp containing dioxins or PCBs. Of course, the main source of contamination for dioxins and PCBs in cultured shrimp is not thought to result from the use of chlorine in hatcheries or production ponds, but from the use of fish oil in the preparation of feed.
Chloramine and chlorine dioxide (ClO2) possibly have advantages over chlorine gas and calcium hypochlorite for use in water containing ammonia and organic matter in appreciable concentrations. Chloramines contain chlorine and ammonia in various proportions (NH2Cl, NHCl2, NCl3). The advantage of chloramine is that it is already combined with ammonia and will not form halogenated organic compounds. Its disinfecting power is as good as that of chlorine, provided there is longer contact time. Chlorine dioxide is equal to or greater than chlorine in disinfecting power. It has an extremely high oxidation potential, which accounts for its potent germicidal powers. Chlorine dioxide residuals and end products are believed to degrade quicker than chlorine residuals. Formation of halogenated organic compounds has not been observed with the use of chlorine dioxide, as has been the case with chlorine and hypochlorites (Tchobanoglous 1991).
Chlorine gas is toxic and a number of extremely bad accidents with numerous deaths have resulted in several countries from the transportation and use of chlorine (White 1992). If containers of this gas are transported frequently within a particular area, a bad accident is almost certain to eventually occur. Also, leaks of chlorine gas can lead to serious consequences for workers, so breathing masks should be available for use by workers.
• Formaldehyde
Formaldehyde solution (or formalin) is a general disinfectant used as a germicide, fungicide, or preservative in various industries. Its main mode of action is to form covalent cross-links with functional groups on proteins. Formalin will cause irritation of the respiratory system and skin reactions in humans. It is suspected to be a carcinogen. In the context of aquaculture, the chemical may be applied to the entire pond volume, but more commonly, treatment is limited to puddles of water in the bottom of ponds after harvest. It is also used as a disinfectant in hatcheries. Formaldehyde is thought to be degraded by natural processes before shrimp are stocked for the next crop (Boyd and Massaut 1999). No food safety hazard is thought to be associated with the use of formaldehyde solution.
• Providone-iodine
The germicidal effect of providoneiodine is based on the action of iodine. This product is marketed as Chemlok l, containing 10 percent providone-iodine and 1 percent of available iodine, or as Argentine. The use of providone-iodine has been approved by the US Food and Drug Administration (FDA) as a fish egg disinfectant. It is commonly used in Ecuador to disinfect larvae and nauplii, by exposing them to 20 ppm of available iodine in a dip batch for 30 seconds. Providone-iodine is thought to be degraded by natural processes in water and should not pose a threat to the environment (Boyd and Massaut 1999). Scientific information is lacking concerning the possibility that this compound or its reaction or degradation products are bioaccumulative.
• Quaternary ammonia compounds
Timsen (n-alquil dimethyl benzil ammonium chloride) and Bromol-50 (containing didecil and dimethyl ammonium bromide) are quaternary ammonium compounds used in hatcheries to disinfect larvae, tanks, and other equipment. These compounds are sometimes added to ponds at 400 grams per hectare in attempts to kill bacteria. Quaternary ammonium compounds disrupt cell membranes and are most active against gram-negative bacteria. There is no information on the possibility that these compounds, their reaction products, or their degradation products are bioaccumulative or pose any threat to the environment.
• Furazolidone and malachite green

Furazolidone is a common antibiotic used by humans as a prophylactic treatment. In aquaculture, it is used to disinfect larvae and as a prophylactic agent during transport. Other antibiotics are also used for the treatment of shrimp disease in hatcheries and grow-out ponds or applied prophylactically to prevent outbreaks of disease. The use of antibiotics in shrimp culture raises several issues of concern to human health, product quality, and the environment. Several studies on salmon farms have shown that antibiotic residues can be extremely persistent in marine sediments, and may lead to the development of bacterial antibiotic resistance. Use of chloramphenicol has caused increased bacterial resistance in shrimp hatcheries in Ecuador (Baticados and Paclibare 1992).
The ecological implications of such antibiotic misuse are not known, but at the very least there is a direct threat to shrimp farming created by the emergence of antibioticresistant pathogens. There is also concern that transfer of such resistance to human pathogens could have serious human health implications. Antibiotics can leave residues in shrimp flesh that may lead to rejection of products in export markets. Residues of the antibiotic oxytetracycline were detected in farm-reared shrimp from Thailand, and caused rejection of shipments to Japan (Macintosh and Phillips 1992). Malachite green is sometimes used in hatcheries in Ecuador to disinfect larvae and nauplii. Its use in aquaculture is prohibited in the USA. Drugs such as Furazolidone and malachite green are implicated as potential carcinogens and allergens (Schnick, 1991).
• Calcium hydroxide and calcium oxide
Calcium hydroxide (hydrated lime) and calcium oxide (burnt lime) are used at a rate of 100 kilograms per hectare in production ponds in an attempt to reduce pathogenic bacteria, by temporarily increasing water pH to 10 or more. Both hydrated lime and burnt lime are strongly caustic and should be handled cautiously. Contact with the eyes can possibly cause blinding, and severe irritations can result from skin contact. If used excessively, these compounds increase water pH up to 10 or more and cause toxicity to aquatic plants and animals, although this risk is low in wellbuffered brackishwater. The water pH will decrease to acceptable levels within a few days after applications, and ponds can be stocked. Liming materials should be stored indoors. Large spills of hydrated lime or burnt lime on land or in water could cause high pH in the surrounding waters. Calcium hydroxide and calcium oxide are not known hazards for food safety (Boyd and Massaut 1999). Some of the calcium will be absorbed by the pond biota to become normal constituents of plant and animals, adsorbed by the soil, or dissolved in the water.
• Plant extracts
Three agents, KILOL, garlic and extract of passion fruit, are used in Ecuador. KILOL is made from extracts of grapefruit seed and contains a mixture of ascorbic acid and large amounts of amino trace elements. It is applied to shrimp ponds, either directly or mixed with lime, and is claimed to be a general water quality enhancer and bactericide. It is also added sometimes to shrimp feed. Both major compounds of KILOL are listed on the GRAS list by the FDA, and are considered generally safe if added to human food. Garlic and extract of passion fruits are also applied to ponds as potential bactericides. These compounds should not cause environmental harm or pose worker safety or food contamination concerns.
Water quality enhancers
The group of chemicals called water quality enhancers comprises products used in attempts to remove ammonia and improve water quality in ponds. The most commonly used are zeolite, KILOL, and probiotics. Zeolite is an aluminosilicate clay of high cation exchange capacity and is applied to shrimp ponds at rates of 20 to 50 kilograms per hectare. Shrimp farmers apply zeolite in an attempt to reduce ammonia concentrations, but it is not effective for this purpose (Boyd 1995). Zeolite will settle to the pond bottom and will not enter the food chain. It does not cause food safety problems or environmental threats. KILOL has already been discussed above. The common probiotics used in pond management are live bacterial inocula (non-pathogenic organisms) and fermentation products rich in extra-cellular enzymes. Examples of probiotics are Biobac, an enzyme solution applied at a rate of 0.5 to 1.0 liters per hectare as a soil and water quality enhancer, and Biostart, a fermentation product applied at 2 to 4 ppm every two days. The mechanisms of probiotic action are not known and the conditions under which improvements may be expected have not been clearly identified. The addition of probiotics to shrimp ponds should not result in any damage to the shrimp crop or to the environment. No food safety hazards are thought to result from probiotics.
Immunostimulants
The last group of agents comprises products used in attempts to boost the shrimp immune system. It includes Chanca piedra (an extract from the plant Phyllantus niruri), vitamin mixes (e.g., Rovimix containing a large array of vitamins and minerals), products containing glucan, probiotics, extracts of natural products (e.g., aleo oil, garlic, extract of passion fruits), and homeopathic products (e.g., Promoter H). In general, these products are mixed with the feed and fish oil is added in the process to assure a stable coating. Their effectiveness in aquaculture ponds still needs to be demonstrated and particularly in the context of WSSV. In general, immunostimulants should not cause any hazard to the environment or food safety problems.
Conclusions
The risk associated with agents used in attempts to control WSSV disease are summarized in Table 1 below.
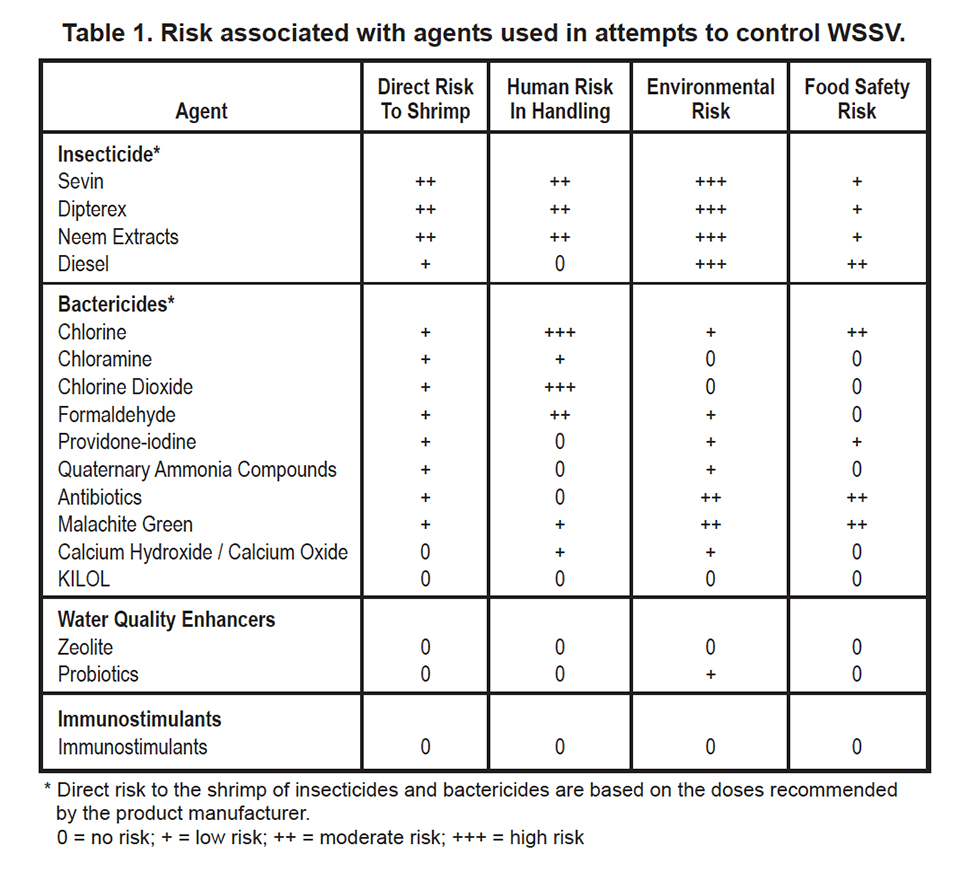
Some aquaculture chemicals may be irritants or toxins to workers who apply them to ponds, and diesel fuel is flammable or explosive. Shrimp farmers are usually aware of the human dangers encountered when handling chemicals, but they often overlook the potential impact of substances on the surrounding environment and on the quality of aquatic food products. Due to increasing concern over the potential harm of aquaculture effluents on receiving water bodies, worries over the contamination of aquatic food products with bioaccumulative and potentially harmful chemicals, and human risks associated with storing and handling some chemicals used in aquaculture, farmers should carefully consider the consequences of using biological and chemical agents in shrimp culture.
It is evident from the extensive literature on insecticides that their use should be discouraged in aquaculture because of their bioaccumulative properties and the high risk of contaminating the environment. Most bactericides are probably degraded by natural processes, but there is a possibility for the development of resistant strains of bacteria through repeated use of bactericides. Also, some bactericides may accumulate in shrimp tissue and pose a food safety problem. Most substances used to improve water quality or to stimulate the immune system of shrimp present little or no risk to the environment or food safety.
Adequate guidelines and regulations regarding chemicals and other agents have not been formulated in most countries. Shrimp farmers who use these substances should follow product labels regarding dosage, withdrawal period, proper use, storage, disposal and other constraints, including environmental and human safety precautions. Also, careful records should be maintained regarding use of chemicals in ponds, as suggested by the Hazard Analysis and Critical Control Point (HACCP) method. A greater effort must be made to prepare lists of acceptable chemicals and recommendations for the use of these chemicals. Some chemicals are necessary in aquaculture, and a system for using them in a safe and publicly acceptable manner must be developed and implemented by the aquaculture industry worldwide.
Cited references on request to authors.
(Editor’s Note: This article was originally published in the June 2000 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
-
-
Claude E. Boyd, Ph.D.
Department of Fisheries and Allied Aquacultures
Auburn University, Alabama USA
Related Posts

Health & Welfare
Assessment of transmission risk in cooked, WSSV-infected shrimp
Exported cooked shrimp infected with White Spot Syndrome Virus (WSSV) and tested positive by PCR is considered a risk factor for the introduction of the pathogen.

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 1
Indian shrimp hatcheries have experienced larval mortality in the zoea-2 stage, with molt deterioration and resulting in heavy mortality. Authors investigated the problem holistically.

Health & Welfare
CENIACUA develops WSSV-resistant shrimp in Colombia
To combat white spot syndrome virus (WSSV) in white shrimp, Corporación Centro de Investigación de la Acuacultura de Colombia (CENIACUA) initiated a selective-breeding program to develop resistance in shrimp.

Health & Welfare
Greenhouse systems a promising technique against WSSV in Ecuador
CENAIM’s greenhouse production trials were carried out at the commercial Pesglasa shrimp farm, located in the Taura region of Ecuador’s Guayas Province.


