Little work has been done on vitamin K in fish
The research on the roles of lipid-soluble vitamins in marine fish larvae is unevenly distributed. A considerable amount of work has been done on vitamin A and some work has been conducted on vitamin E, while studies on vitamins D and K are virtually lacking in the literature.
Knowledge of the principal functions of nutrients may, however, be extrapolated from studies on larger fish or even from mammalian studies, but future studies must verify that this knowledge is valid in the fish larvae context.
Vitamin A
Vitamin A is essential for directing the development of organ and body axes in vertebrate embryos, and the author believes the vitamin may have a similar function in developing fish larvae. Retinoic acid is the active form of vitamin A participating in these processes.
Specialized cell clusters that produce retinoic acid develop in a strategic area of the organ or embryo. The retinoic acid diffuses over the tissue, setting up a gradient in which the different cells react to their specific retinoic acid concentrations, deciding the pattern of cell proliferation and differentiation.
To regulate the proliferation and differentiation of cells, retinoic acid binds to nuclear receptors that bind to DNA and stimulate or inhibit the gene transcription of a large number of downstream genes. This regulation of gene transcription is often dependent on interactions with other nutrients and hormones, which have specific nuclear receptors such as vitamin D, fatty acids and thyroid hormones. Vitamin A is also important in vision, where the active form, retinal, forms a reversible complex with rhodopsin in the light-sensitive cells in the eyes.
Optimum levels for halibut juveniles
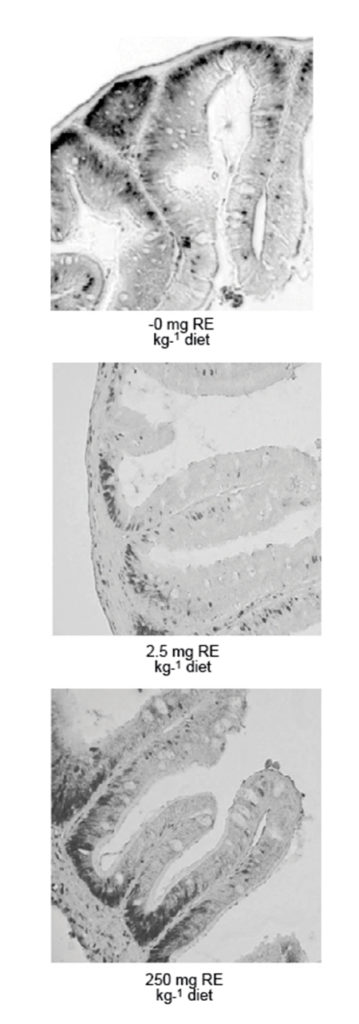
The National Institute of Nutrition and Seafood Research in Bergen, Norway, has used proliferation and differentiation of enterocytes to determine the optimal level of vitamin A for Atlantic halibut juveniles with initial weight of 0.4 grams. The growth data indicated a requirement of 0.75 mg vitamin A/kg dry diet, which is the same as the recommendation by the National Research Council.
However, when staining enterocytes with an antibody against a protein that is only present in dividing cells, researchers found that cells were proliferating only in the crypts of the intestine (normal situation) when the fish were given 2.5 mg/kg vitamin A. At higher or lower concentrations, cells that had migrated out of the crypts were also proliferating. Thus, cells were proliferating heavily at too-high or too-low dietary vitamin A levels.
A reverse picture, with more differentiated cells at 2.5 mg/kg vitamin A, was seen when the brush border enzymes of the enterocytes were analyzed, assuming that highly differentiated cells have high levels of these enzymes.
The author concluded that the optimal dietary level of vitamin A for small Atlantic halibut juveniles is 2.5 mg/kg. It is not certain that larvae have the same optimum, but there is no documentation that the requirements are different in fish that are so close in age as these young juveniles and larvae.
Pigmentation in flounders
Japanese scientists have shown that enrichment of rotifers and artemia with high levels of vitamin A stimulated pigmentation in Japanese flounders, but at the same time gave rise to extensive skeletal deformities. This showed that the mechanisms regulating pigmentation were influenced by vitamin A, but that the levels needed for stimulation were toxic to the animals. Pigmentation was not stimulated by high levels of vitamins D and E.
Copepods, artemia and rotifers contain very little vitamin A, but fish larvae appear to be able to convert their astaxanthin and cantaxanthin to vitamin A. Copepods and artemia contain high levels of astaxanthin and cantaxanthin, apparently sufficient to cover the vitamin A requirement in fish larvae. Rotifers need to be enriched with vitamin A.
Vitamin E
The primary function of vitamin E is to protect organisms against lipid oxidation. Lipid oxidation, also called autooxidation, is a chain reaction which starts with the reaction of a free radical with a polyunsaturated fatty acid. This eventually gives a fatty acid radical that can react with a new fatty acid, and a new cycle is started.
Vitamin E acts as a chain-breaking antioxidant, neutralizing the fatty acid radicals. In the process, vitamin E is oxidized to the vitamin E radical, which is relatively stable and can be regenerated to vitamin E by ascorbic acid. The ascorbic acid radical is regenerated, ultimately by reducing equivalents obtained from energy metabolism.
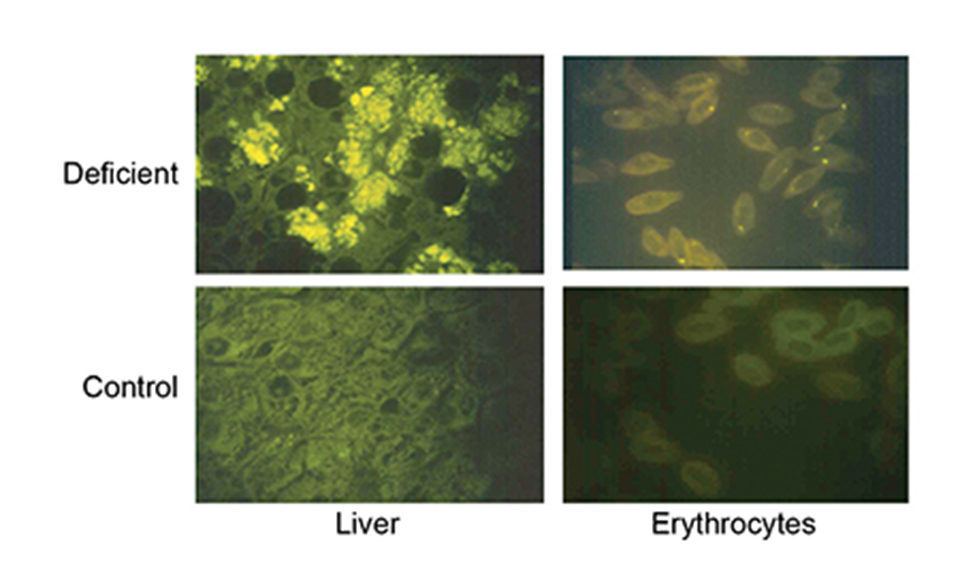
Vitamin E is thus part of a complex system of exogenic and endogenic antioxidants. The primary symptom of vitamin E deficiency is accumulation of lipid oxidation products in the tissues that can lead to pathologies such as liver and muscle degeneration and anemia, and ultimately reduced growth and survival.
Vitamin interactions
Marine fish larvae are probably subjected to high levels of oxidative stress. Live feed production and enrichment is performed under highly pro-oxidative conditions, with high levels of omega-3 polyunsaturated acids, air or oxygen addition to the culture water, high temperature and bright light. Formulated diets for marine fish larvae also contain high levels of polyunsaturated fatty acids and pro-oxidants, for example, in the form of minerals. The high surface:volume ratio of the feed particles also favors oxidation.
It is therefore important to supplement marine fish larval diets with vitamin E, but vitamin E at high levels in the absence of sufficient vitamin C can increase mortality and tissue lipid oxidation in Atlantic salmon and halibut juveniles, and sea bream larvae. The concentration ratio of the two vitamins in copepods (110 mg kg-1 vitamin E and 500 mg kg-1 vitamin C) may give a guideline for supplementation of larval diets.
Interactions between dietary docosahexaenoic acid (DHA) and vitamin E, and between vitamins C and E have been shown in marine fish larvae and young juveniles, respectively. Feeding a high level of DHA, which is the fatty acid most susceptible to oxidation, to seabass larvae combined with a low level of vitamin E led to muscular lesions in the larvae. However, an increase in vitamin E or decrease in DHA level reduced the rate of muscular damage.
In Atlantic halibut juveniles, mortality due to vitamin C deficiency was greater at higher than at lower levels of vitamin E. This was also seen in Atlantic salmon juveniles. Conversely, salmon showed decreased mortality due to vitamin E deficiency when the dietary vitamin C level was increased. This showed that the requirement for vitamin E depends on the composition of the diet.
The requirement for vitamin E in marine fish larvae is not known, but the general opinion is that vitamins C and E should be fed at the g/kg level. The NRC gives requirements for vitamins C and E at 30 and 50 mg/kg, respectively, for larger fish. The reason for adding high levels of these vitamins to larval diets is that the literature gives some indications that high levels protect against stress. There is also a motivation to add vitamin E to prevent oxidation in larval diets.
Vitamin D
Vitamin D participates in regulating calcium and phosphorus homeostasis in vertebrates, including absorption of these minerals from the diet and water, and deposition and resorption of bone. The active compounds are two hydroxylated forms of vitamin D, 1,25-dihydroxyvitamin D3 (calcitriol) and 24,25-dihydroxyvitamin D3.
When calcium and phosphorus are available, vitamin D stimulates bone mineralization. During starvation or limited supply of bone minerals, vitamin D stimulates bone resorption. Studies on vitamin D have not been performed on marine fish larvae, but vitamin D and calcitriol were shown to stimulate bone formation in zebrafish yolk sac larvae.
Vitamin K
Vitamin K is a cofactor in carboxylation of glutamate in protein that becomes bone proteins or blood coagulation factors. One of the bone proteins is osteocalcin, which binds calcium and therefore is important for bone mineralization.
There is interaction between vitamins D and K in that vitamin D induces the synthesis of osteocalcin. Little work has been done on vitamin K in fish, and studies on marine fish larvae are absent. However, two studies on mummichog showed that failure to supplement broodstock and larvae with vitamin K may cause thin and weak bones, both in early development and during growth, and induce bone structure abnormality.
Since bone deformities, some of them probably originating from the larval stage, are very common in farmed marine fish, it is important to study the roles of vitamins D and K during the larval stages of marine fish larvae.
(Editor’s Note: This article was originally published in the November/December 2008 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Author
-
Dr. Kristin Hamre
National Institute of Nutrition and Seafood Research
P. O. Box 2029
Nordnes – 5817 Bergen, Norway[111,110,46,115,101,102,105,110,64,97,104,107]
Tagged With
Related Posts

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Aquafeeds
A look at phospholipids in aquafeeds
Phospholipids are the major constituents of cell membranes and are vital to the normal function of every cell and organ. The inclusion of phospholipids in aquafeeds ensures increased growth, better survival and stress resistance, and prevention of skeletal deformities of larval and juvenile stages of fish and shellfish species.

Health & Welfare
AHPN inferences based on behavior of vibrio bacteria
Vibrio parahaemolyticus, a strain of which is the cause of acute hepatopancreatic necrosis (AHPN), has both virulent and benign strains. This strain colonizes the stomachs of shrimp by the formation of a biofilm, which protects it from antibiotics and other potential treatments.

Health & Welfare
Achotines laboratory home to continuing studies of tuna early life history
The Inter-American Tropical Tuna Commission Achotines Laboratory in southern Panama is the world’s only facility with nearly year-round availability of tuna eggs and larvae. A study is comparing the reproductive biology, genetics and early life history of yellowfin and Pacific bluefin tuna.


