NMFS researchers developing diets, hatchery technology

The sablefish (Anoplopoma fimbria) also known as black cod, is a high-value species found off the Pacific coast of mainland United States and the U.S. state of Alaska. Sablefish have been identified as a priority species for commercial farming in the Pacific Northwest, where they are the target of a restricted commercial and recreational fishery.
Their firm, oily flesh demands up to $10/kg at the dock, making sablefish one of the highest per-weight value fish in the region. With wild catch quotas dropping, cultured sablefish will no doubt play an increasing role in the market.
The National Marine Fisheries Service Manchester Research Station in Port Orchard, Wash., USA, has been conducting research to develop a viable aquaculture system for the production of sablefish using conventional net pens, tank-based systems and offshore cage grow-out technology. With assistance from Troutlodge Marine, the world’s largest producer of trout eggs, work to date has focused largely on the development of diets and hatchery technology.
Sablefish growth, nutrition
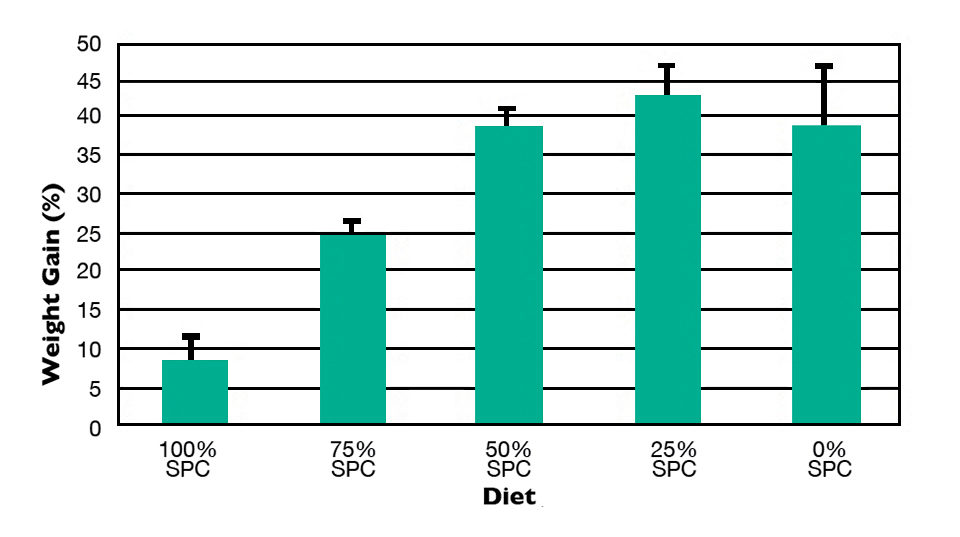
Little is known about the specific dietary needs of sablefish, but it would be beneficial if sablefish could be grown with minimal fishmeal and fish oil, which are expensive and in limited supply. Feeding trials in the authors’ lab demonstrated that sablefish can grow well on a diet with up to 50 percent of the fishmeal replaced with soybean protein concentrate (Fig. 1). Weight gain was not significantly less than that for fish fed a diet with 100 percent fishmeal.
In the wild, sablefish generally obtain heart-healthy fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) by eating organisms that have accumulated the fatty acids through the food web. These fatty acids are not present in terrestrial plants, and researchers are unsure whether sablefish can directly synthesize EPA and DHA from vegetable oils.
Compound feeds in which fishmeal is replaced by corn, soy and wheat products, and fish oil is replaced by vegetable oil are being tested with sablefish at the Manchester Research Station. The authors are currently determining the effects of fish oil replacement on feed consumption, fish growth and the fatty acid composition of the fillets. Future studies will determine how much EPA and DHA must be added to the diets and, if needed, test various sources obtained from marine algae.
Broodstock spawning
Photoperiod manipulation is being used to induce fish to spawn throughout the year at the Manchester Research Station hatchery. In the wild, sablefish generally spawn during a narrow window dependent on temperature and light. These factors can be manipulated to provide spawning adults at intervals convenient for researchers or the hatchery.
The authors first started working with sablefish in 2002. During the 2002 season, three populations of sablefish totaling about 90 fish were switched to three unique photoperiods: advanced, delayed and natural. Approximately 10 percent of the fish in the advanced group spawned prior to the beginning of the natural spawning season, and none spawned during the natural season. A similar number of fish from the other two photoperiods spawned during the season predicted by the controlled photoperiods.
Female sablefish rarely spawn on their own in captivity, so spawning induction is necessary to produce eggs. Spawning induction has been partially successful by the use of slow-release hormonal implants made in the lab or a commercial product.
After a break of seven years, the authors are currently spawning fish during the natural season. They are also putting additional fish into regimes that should result in spawning for nine months of 2010.
Determining maturation status
The Manchester Research Station uses ultrasound to track the development of the ovaries and testes in sablefish to determine the maturity status of each fish and minimize the number of invasive biopsies. This is accomplished by tagging the fish with microchips and sampling every two months with ultrasound. Measurements of the gonads can be done using the ultrasound unit itself or image analysis software on a computer.
Both males and females progressing toward spawning show an increasing gonadal cross section. Measurement of this cross section has the same application as the gonado-somatic index typical of fisheries management without the need to sacrifice the fish. Injections or implants of gonadotropin-releasing hormone are needed to induce final maturation and ovulation.
Typically, the timing of hormone delivery is based on oocyte size. An oocyte diameter of 1.1 mm is the minimum size at which hormone treatment has been successfully administered in the lab. Oocyte measurement is typically achieved by taking a suction biopsy of the ovary. However, this is invasive and can result in tissue damage and infection, and the associated stress could disrupt the natural progression to ovulation. Ultrasound offers a less invasive technique to determine maturation in sablefish and guide the timing of hormone implants.
Determining the sex of unripe sablefish based on external morphology is also difficult, but it is possible to determine sex using ultrasound. While there is a learning curve involved, differentiating males and females is relatively straightforward. Future studies will include optimizing the time to implant hormones based solely on gonad cross section images.
Larval rearing
Females are monitored closely for rapid weight gain. Sablefish are batch spawners whose eggs become ready every 48 hours. If the spawning time is missed, the egg viability is reduced. Eggs are fertilized and checked at the four-, eight- and/or 16-cell division with a stereoscope to determine fertilization. Fertilized eggs are placed in funnel-shaped 200-l upwelling tanks for 11 days at 6 degrees-C until the tails emerge.
Sablefish have a tailed, nonfeeding embryo stage that lasts approximately 25 days. Just before hatching, they are transferred to 600-l upwelling cones. Once they begin feeding, they are moved to tanks and receive a combination of enriched rotifers and Artemia. Once beyond metamorphosis, sablefish juveniles eat aggressively and grow rapidly.
The authors are now evaluating several key areas of larval rearing, including fertilization methodologies and egg incubation procedures. Studies to determine optimal egg:milt ratios, densities and optimal temperature and salinity parameters for incubation are also being conducted. Several incubator types will be compared once the optimal water quality parameters are defined.
Long-term goals
For sablefish farming to become a reality, further efforts to develop technologies to produce fingerlings, formulate cost-effective feeds from sustainable sources and design efficient growout systems are critical. In addition, cultured sablefish must meet or exceed the quality of wild sablefish.
The authors’ long-term goals for the sablefish program include the development of a pilot-scale photoperiod-controlled recirculating juvenile production system, as well as continued research on methods for incubating eggs and larvae, and formulating sustainable feeds. They also plan to monitor environmental effects and develop growout technologies for traditional net pens, offshore cages and land-based systems that will transfer to other researchers and businesses.
(Editor’s Note: This article was originally published in the May/June 2009 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Matthew A. Cook
National Marine Fisheries Service
Manchester Research Station
7305 Beach Drive East
Port Orchard, Washington 98366 USA -
Tom Wade
National Marine Fisheries Service
Manchester Research Station
7305 Beach Drive East
Port Orchard, Washington 98366 USA -
Kenneth Massee
National Marine Fisheries Service
Manchester Research Station
7305 Beach Drive East
Port Orchard, Washington 98366 USA -
William Fairgrieve
National Marine Fisheries Service
Manchester Research Station
7305 Beach Drive East
Port Orchard, Washington 98366 USA -
Eric Kroeger
National Marine Fisheries Service
Manchester Research Station
7305 Beach Drive East
Port Orchard, Washington 98366 USA -
Michael B. Rust, Ph.D.
National Marine Fisheries Service
Manchester Research Station
7305 Beach Drive East
Port Orchard, Washington 98366 USA
Tagged With
Related Posts

Innovation & Investment
Aquaculture in Canada: status, perspectives
Canada exports farmed seafood products to more than 22 countries and is the main seafood supplier to the U.S. market. Finfish, primarily salmon, production is strong and shellfish production is growing, but diversification will be imperative to maintain competitiveness.

Innovation & Investment
Aquaculture Exchange: Barry Costa-Pierce, UNE
University of New England Professor Barry Costa-Pierce says aquaculture is often neglected in studies examining ocean health and ecosystem and resource management. The “Ocean Prosperity Roadmap” released this summer, he said, was more of the same.

Aquafeeds
A look at corn distillers dried grains with solubles
Corn distillers dried grains with solubles are an economical source of energy, protein and digestible phosphorus to reduce feed costs and fishmeal usage.

Aquafeeds
A new nutrient for aquaculture, from microbes that consume carbon waste
Biotechnology firm NovoNutrients aims to produce a line of nutraceutical aquafeed additives as well as a bulk feed ingredient that can supplement fishmeal. Its process includes feeding carbon dioxide from industrial gas to a “microbial consortium” starring hydrogen-oxidizing bacteria.


