Polyhydroxyalkanotes are a family of biodegradable biopolymers produced from sugar fermentation

Nitrate, the end-product of nitrification, has historically not been of major concern in recirculating systems due to its low toxicity to freshwater organisms. However, with the inherently high degree of water reuse in marine recirculating aquaculture systems, nitrate reduction becomes critical due to the compound’s toxicity to marine organisms from its effects on the animals’ osmoregulatory abilities. These effects can be manifested in the inhibition of reproductive cycles, poor egg development, delayed hatching times, reduced growth rates, and higher mortalities.
Additionally, nitrate is very stable in the natural environment and can be a source of pollution in waters that receive aquaculture effluent. Nitrates can cause eutrophication and algal blooms, and resulting high dissolved-oxygen consumption as the algae die off.
The United States Environmental Protection Agency has limited the nitrate and nitrite concentrations in potable water to 10 and 1 mg per liter, respectively, and placed their control on the priority list. While denitrification can be expensive, at some point in time, it will be mandatory for facilities with limited water supplies or stringent discharge requirements.
Denitrification
Denitrification is the process in which nitrate is oxidized to nitrogen gas and removed from water. This process can be optimally mediated by denitrifying bacteria under specific conditions, such as low redox potential, low oxygen levels, availability of organic carbon and nitrate sources, pH of 7.0 to 8.5, and a temperature range of 25 to 32 degrees-C.
Denitrification achieved by adding water-soluble carbon sources such as methanol, ethanol, or acetic acid is a traditional tool used by the waste treatment industry to reduce nitrogen pollution. However, sophisticated and costly computer control systems are often required to properly regulate the carbon dosage.
In the absence of nitrate in an anaerobic environment, excess carbon can reduce the redox potentials, promoting the reduction of sulfates and production of toxic sulfides. Denitrification methods also require multiple treatment components, further increasing the overall cost.
Alternative carbon source
In 2006, Aquaculture Systems Technologies, LLC (AST) received a Phase I U.S. Department of Agriculture Small Business Innovation Research Program (SBIR) project to investigate the potential for using polyhydroxyalkanotes (PHAs), a family of biodegradable biopolymers produced from sugar fermentation, as an alternative carbon source for denitrification in recirculating aquaculture systems.
The biodegradation of polyhydroxyalkanotes in the presence of nutrients releases organic carbon, which makes them an ideal substrate for self-regulating, passive denitrification reactors. PHAs offer a potential low-maintenance and cost-effective denitrification method since they act as both carbon sources and substrates for denitrifying bacteria. This, in turn, eliminates the need for the sophisticated control systems and handling of hazardous chemicals required by conventional methods of treatment.
Fouling solution
Earlier research showed the excellent denitrification capability of PHAs, but excessive biofloc formation from the heterotrophic bacteria using the carbon released from the PHAs fouled the columns and limited the process.
The authors hypothesized that the problems associated with clogging of the PHA filter bed could be addressed by utilizing AST’s patented PolyGeyser bead filter technology as a denitrification platform. This bead filter promotes a healthy thin biofilm due to its frequent, gentle backwashing, which eliminates the clogging problem observed with packed beds. Additionally, the ability to manipulate backwash frequency allows the biofilm abrasion rate to be tuned.
Lab study
A lab-scale denitrification system was built with three units that utilized the same design criteria used for the commercial bead filters. Numerous replicated tests were conducted to quantify the denitrification capacity of the PHAs and the impact of backflushing on performance. For each test, water quality samples of influent and effluent were analyzed for pH, ammonia-nitrogen, nitrite-nitrogen, nitrate-nitrogen, and alkalinity. Dissolved oxygen and temperature were also measured in each bioreactor and the production tank.
In order to characterize the removal rate across the media bed, samples were taken at several depths in the media bed using a syringe with a long stainless steel needle. Samples were taken from below the media, at the bottom, at three locations across the bed, at the top, and finally at the effluent.
Results
In one such test at a flow rate of 150 ml per minute and with 2 liters of PHA media, the dissolved oxygen levels across the media bed fell quickly from approximately 3.1 mg per liter at the bottom of the media to less than 0.6 mg per liter just above the top. Although the dissolved oxygen content remained above 2.0 mg per liter across 50 percent of the media bed, alkalinity, a byproduct of the denitrification process, increased fairly uniformly across the bed (Fig. 1). The alkalinity increased from 188 to over 240 mg per liter as calcium carbonate, and correspondently, the nitrate-nitrogen levels fell from approximately 100 to 78 mg per liter.
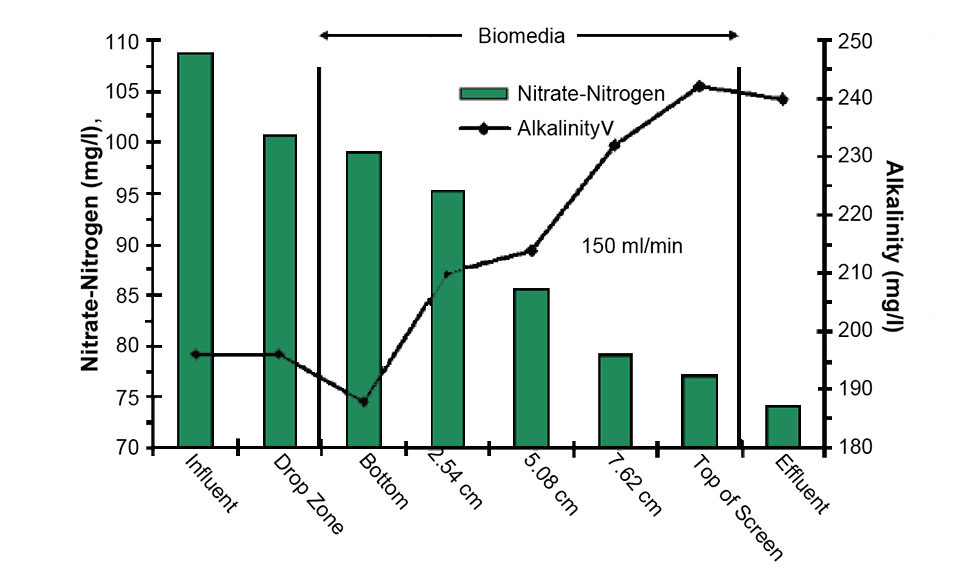
The SBIR research showed that removal rates for nitrate by the lab-scale bioreactor units surpassed 2 kg per cubic meter daily with no clogging by biofloc or short circuiting of the media bed. Backwashing to maintain the biofloc was performed every one to two days. Excess biofloc settled quickly to the bottom of the bioreactors and was drained from the system on a daily basis.
(Editor’s Note: This article was originally published in the May/June 2007 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
James M. Ebeling, Ph.D.
Aquaculture Systems Technologies, LLC
P.O. Box 15827
New Orleans, Louisiana 70175 USA -
Pradyot Deshpande
Aquaculture Systems Technologies, LLC
P.O. Box 15827
New Orleans, Louisiana 70175 USA -
Douglas G. Drennan II
Aquaculture Systems Technologies, LLC
P.O. Box 15827
New Orleans, Louisiana 70175 USA -
Patrick G. Simms
Metabolix, Inc.
Montgomery, Louisiana, USA
Tagged With
Related Posts

Responsibility
Aquaponic system produces red drum, saltwater vegetable species
A project in Florida is studying the feasibility of a marine aquaponic system containing red drum and two native saltwater species. Water that exits the plant raceways is filtered and recirculated to the fish tanks. In tests, sea purslane grew rapidly, while saltwort took almost four months to adapt. The fish exhibited high survival and achieved a feed-conversion ratio of 1.2.

Responsibility
Bacterial amendments in shrimp grow-out ponds
Pond microbial communities are a critical and often overlooked component of aquaculture ecosystems. Bacterial amendments like probiotics provide significant support to shrimp farmers around the world.

Health & Welfare
Beneficial microbes and pathogen control
A range of alternative products is available to improve animal health and water quality, and control pathogen loads. Beneficial microbes produce antimicrobial compounds and suppress pathogen proliferation.

Health & Welfare
Biofilter inoculation in recirculating aquaculture systems
Biological filters are essential parts of recirculating aquaculture systems that transform toxic fish compounds such as ammonium and nitrite into less-harmful nitrate. The authors tested the convenience and efficiency of three methods for the initial inoculation of aerobic biofilters.


