Industry needs tools to cope with current viral epizootic and worry less about preventing epizootics
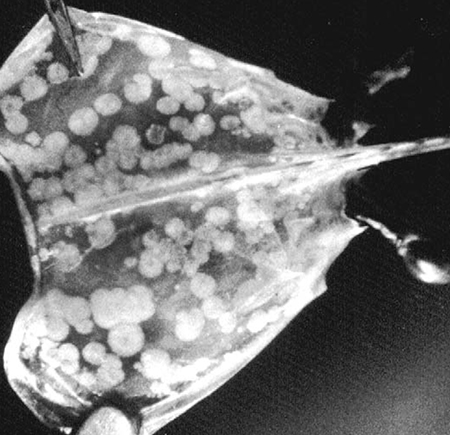
Shrimp culture production increased rapidly during the 1980s but reached a plateau at around 700,000 tons per year during the 1990s, due primarily to diseases. Viruses have been the etiological agents most responsible for these devastating losses, and no country with a significant shrimp farming industry has escaped. Since 1994, global losses of farmed shrimp due to viruses have been estimated as high as $4 billion.
Losses to shrimp diseases should come as no surprise, for such has been the fate of most agricultural ventures. What is surprising is that these enormous losses have been met with such little effort directed toward finding solutions. A few groups, led by Drs. Donald Lightner (University of Arizona, USA) and Tim Flegel (Mahidol University, Bangkok, Thailand) have made considerable progress in identifying, characterizing and detecting the viruses. However, little effort has been made toward understanding the defensive capabilities of the shrimp and the mechanisms they use to protect themselves against infection. The fact that shrimp do not possess an immune response analogous to the vertebrate antigen/antibody reaction does not mean that they have no defenses.
There are seven recognized, problematic shrimp viruses: Baculovirus Penaei (BP), Baculoviral Midgut Gland Necrosis Virus (BMN), Infectious Hypodermal and Hematopoietic Necrosis Virus (IHHNV), Hepatopancreatic Parvo-like Virus (HPV), Monodon Baculovirus (MBV), Taura Syndrome Virus (TSV), White Spot Syndrome Virus (WSSV) and Yellowhead Virus (YHV). We do not know how widespread these viruses are in nature, or the ability of these viruses to jump from one species to another. However, it is likely they are much more widespread than has been reported.
Viral entry, replication, And transmission
Dr. T.W. Tinsley (with the Natural Environment Research Council of Invertebrate Virology, Oxford, England) suggests that the portal of entry for viruses into insects could be expected to be at any point along the route from the mouth to the gut tissue. Other scientists have suggested that the midgut epithelial cells are the focal entry point for all virus groups associated with insects, and that receptor binding sites possibly act as a “gut barrier” to the entry of virus into the shrimp’s circulatory system (hemocoele).
Unlike bacteria, viruses cannot multiply outside a host cell. Once the virus enters a host, it infects susceptible cells (if the host possesses receptor sites that match the receptors on the surface of the virus). Enzymes then attack the cell wall, allowing the virus to inject its DNA or RNA into the cell, where it replicates and produces many copies of itself. Shrimp viruses liberated from a host cell have a relatively short life span in water or air, unless they are protected in some manner such as freezing or desiccation, or by certain organic compounds. This limits spread unless animals are in close proximity to each other. A study conducted by Grupo Granjas Marinas of Honduras indicated that free TSV particles lost their ability to infect after about five to seven days when held in a clean saltwater environment. Dr. Jeff Lotz (Gulf Coast Research Laboratory, Ocean Springs, Mississippi) found the virus capable of infecting early stage juveniles for as long as 21 days when placed in a contaminated environment, presumably being protected by an organic film.
In my opinion, there is little chance of preventing the spread of viruses among neighboring countries. There are no barriers along coastal waters common to Central and Latin American countries that would prevent transmission via migrating shrimp, birds and other possible hosts and vectors of pathogenic viruses. Annual world trade of thousands of tons of frozen shrimp and the ship traffic from hemisphere to hemisphere – with the taking on and discharging of ballast from port to port – will insure that the viruses will eventually find their way into all but the most biosecure farms. And besides, viruses come from wild populations and a “new” virus is as likely to be identified in one country or region as another.
Diagnosis
With the development of gene probes and PCR specific for nucleic acid sequences unique to the virus from which it was prepared, a common misconception has arisen. This is the belief that a positive reaction signifies the presence of the exact strain of virus from which the probe originated. While this may be true, it is also possible for closely related viruses to share in common one or more nucleic acid base pairs. Without additional sequencing, confusion over the virus’ true identify may occur, falsely identifying a closely related strain of virus. This becomes important when cultivating non-native shrimp. It is assumed that the virus is also an exotic strain leading to a costly mistake, causing the destruction of thousands of dollars of shrimp stock. This is especially true if the virus is innocuous and the infected shrimp are asymptomatic. An example of this appears to be the case involving IHHNV-infected animals at the Harbor Branch Oceanographic Institution (HBOI) in Florida.
HBOI and some of their industrial partners have cultured SPF strains of P. vannamei since 1992, under strict biosecurity measures. A rigorous testing program for shrimp pathogens has been in effect since the beginning. Prior to the development of gene probes and PCR methods of analysis, bioassays and routine histology were used to determine the presence of disease. During 1994, using a gene probe, an isolated group of shrimp tested positive. However, the animals were asymptomatic (without clinical evidence of the disease, such as deformities and/or stunting). Comparative growth studies showed no difference in growth rate.
Histological preparations were made on animals found positive by PCR and stained using an in situ gene probe and routine H&E staining. The results were markedly different from animals that exhibit the classical form of IHHNV. Only hematopoietic tissue from PCR-positive, asymptomatic animals reacted with the in-situ gene probe. Also, H&E stained sections were free of Cowdry Ainclusion bodies.
The virus is difficult to transmit via oral dosing but is readily transmitted by injection. Transmission electron microscopy studies are currently underway. It is important to mention that local grass shrimp (Palaemonetes spp.) have tested positive with the gene probe and are being reevaluated with PCR, following Dr. Jim Brock’s report (Hawaii Department of Agriculture) of false positives with the gene probe when using tissue samples.
Shrimp resistance

As previously mentioned, shrimp do not possess an antigen/antibody immune response analogous to that of vertebrates. Whether or not some other type of a specific recognition system exists, at least for viruses, is cause for debate. During an U.S.-Asian conference held in Hawaii in the early 1990’s, considerable attention was given to the devastating effect that IHHNV was having on shrimp culture in the USA. I reported on the occurrence of IHHNV on six farms in Honduras but could not correlate Runt Deformity Syndrome (RDS), which was almost non-existent, with the number of animals positive for IHHNV or the prevalence of Cowdry A inclusions identified in individual shrimp. I believe this was due to the co-existence of the virus and the shrimp for a considerable length of time, having identified IHHNV in populations of shrimp in both Panama and Honduras several years before.
At the IV Central American Symposium on Aquaculture (Tegucigalpa, Honduras, April 1997), I reported on TSV or a TSV-like infection in shrimp collected from a farm in Colombia in February 1990. At the time, most nauplii and postlarvae used to stock farms in the country originated from Panama hatcheries. My belief that the virus may have been in Panama in the 1980’s was further supported by a report from Mr. Bill More, Manager of Agromarina de Panama. He submitted archived samples of diseased shrimp from 1988 to Dr. Lightner for TSV analysis. His report identified the presence of TSV lesions in those animals. Heavy losses in the nursery ponds were being recorded at that time and were thought to be due to Vibrio infections (known locally as Sea Gull Syndrome). Perhaps this is one of the reasons that Panama animals are in demand in Honduras, having had time to develop a measure of resistance to TSV.
What about the role of genetics, genetic selection and breeding for specific pathogen resistance? Various companies and institutions have aggressive programs to breed SPF shrimp, and this certainly is a logical approach that will undoubtedly have its successes. What has been a relatively slow process with vertebrates will likely be shortened in shrimp culture, due to their relatively short life cycle and fecundity. But these are not the complete answer. Resistance to one virus does not protect against another, and a mutation or two in a virus may render a resistant animal into a susceptible one.
Viral-induced apoptosis and accommodation theory
Dr. Tim Flegel has suggested that the explanation for acute mortality of shrimp populations on initial exposure to viral disease and their increasing tolerance to those diseases over time is explained by apoptosis or cell in response to an acute infection. He has reported on several occasions that susceptible black tiger shrimp (Penaeus monodon) populations challenged with WSSV or YHV during their larval stages have significantly improved survival than those challenged later. He suggests this is due to what he refers to as an “active viral accommodation theory.” According to Dr. Flegel, if the virus is present before the larvae can mount a defense, the virus will be recognized as self and an inflammatory response will not be triggered later by the virus. He suggests that this is unique to viruses and does not apply to bacteria or fungi, and presents a challenging argument.
Possible role of receptor blocking
During the replication of thousands of copies of a virus within a cell, numerous copies will be “deficient” and incapable of infecting another cell. However, these virus particles may attach to cell receptors effectively blocking entrance of “complete” virions, serving as a natural “vaccine” and protecting against lethal infection.
During TSV studies at Sea Farms in Honduras, we noticed that early exposure of shrimp postlarvae improved their survival when challenged with an LC50 dose. I theorized this may have been due to the binding of nonviable virus particles – rendered so by the hemolymph – to newly developing receptor sites on cells that would accommodate active virions when animals were exposed at a susceptible age, thus effectively blocking entrance. Dr. Flegel concedes that receptors may be involved but suggests that they may be part of a specific viral recognition system. In either case, the results suggest that a properly designed vaccine could provide effective protection.
Conclusions
This document is intended as a challenge to researchers and their institutions, universities, and governments to increase studies of shrimp viruses and shrimp immune systems. This should be an effort to provide management with tools to cope with current viral epizootic and worry less about preventing epizootics. Immediate studies are needed on the distribution of shrimp viruses in nature, variations in viral strains within and between host species, and the ability of the virus to jump from one host to another. With the analytical tools (DNA sequencing, gene probes, etc.) now available to researchers, it is little short of a crime that so little funding has been made available to qualifying institutions.
This article is adapted from the presentation by Dr. Laramore at Aquaculture Venezuela ‘99, the Second South American Aquaculture Congress held on November 17-20, 2000 in Puerto La Cruz, Venezuela. in Puerto La Cruz, Venezuela.
(Editor’s Note: This article was originally published in the June 2000 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Author
-
Rolland Laramore, Ph.D.
Technical Director
Aquatic Animal Health and Shrimp
Nutrition Laboratory
Harbor Branch
Oceanographic Institution
Ft. Pierce, Florida USA
Tagged With
Related Posts

Aquafeeds
Analyzing aquaculture through Alltech’s Global Feed Survey
Alltech’s eighth annual Global Feed Survey, a compilation of estimated feed production data, reported that aquafeeds grew globally by 4 percent.

Health & Welfare
Evolutionary history of Taura Syndrome Virus
Based on a phylogenetic analysis with the BEAST program, the authors determined the evolutionary relationships among 83 Taura syndrome virus isolates.

Health & Welfare
Latin America FAO project establishes hatchery standards, info system
At a United Nations Food and Agriculture Organization (FAO) workshop 14 shrimp-producing countries discussed strategies for controlling shrimp diseases.

Intelligence
We can grow better shrimp, and in better ways
The recent Central American Aquaculture Symposium in Choluteca, Honduras, brought together more than 600 participants to discuss industry issues and perspectives. The focus was shrimp diseases and their impacts on production, as well as practical alternatives to face these issues and move forward.


