Method is a valid alternative to salinity measurement by refractometry

Most aquafarmers are accustomed to measuring the salinity of their production systems with salinity refractometers. The refractive index of water changes in a predictable way with increasing salinity. Salinity refractometers are calibrated so they provide direct readings of salinity.
Salinity refractometers provide fairly accurate readings in water with 5 ppt salinity and above. However, they are less accurate at lower salinities. With shrimp culture in low-salinity water becoming common in many areas, more accurate ways of measuring low salinity will be necessary.
Conductivity and salt content
The ability of water to conduct electricity increases as the total concentration of dissolved ions increases. Thus, the measurement of conductivity provides a convenient way of estimating the salt content of water.
Conductivity can be determined accurately with concentrations of ions ranging from those of dilute freshwater to those of full-strength seawater. These measurements can be made in situ quickly and accurately with portable conductivity meters, or water samples can be taken to a laboratory for conductivity measurements with bench-top conductivity meters (Fig. 1).
Specific conductance
Conductivity meters measure specific conductance, which is defined as the conductance afforded by a cubic mass of water with edges 1 cm long. Resistance in electrical circuits is reported in ohms. Conductance, the reciprocal of resistance, is reported as 1 per ohms.
Thus, specific conductance could be given as 1 per ohms-cm, but for convenience, it usually is given as mhos per centimeter. (Note the mho is “ohm” spelled backwards.) A 0.0100 normal (N) potassium chloride solution* at 25 degrees C has a specific conductance of 0.001413 mho per centimeter.
*A 1.00 N solution of a substance contains 1 g equivalent weight of the substance per liter. In the case of potassium chloride (KCl), 74.55 g KCl per liter distilled water gives a 1.00 N solution.
To avoid small decimal numbers, it is customary to report specific conductance as micromhosper centimeter. A 0.0100 N potassium chloride solution has a specific conductance of 1,413 µmhos per centimeter at 25 degrees-C. Because potassium chloride is used as a standard for specific conductance, the relationship between potassium chloride concentration and specific conductance is illustrated in Table 1.
Boyd, Concentration versus specific conductance, Table 1
| Potassium Chloride Normality | Potassium Chloride mg/l | Specific Conductance (µmhos/cm) |
|---|
Potassium Chloride Normality | Potassium Chloride mg/l | Specific Conductance (µmhos/cm) |
|---|---|---|
| 0.0 | 0.0 | 0.0 |
| 0.0001 | 7.5 | 14.9 |
| 0.0005 | 37.3 | 73.9 |
| 0.001 | 74.6 | 147 |
| 0.005 | 373 | 718 |
| 0.01 | 746 | 1,413 |
| 0.05 | 3,728 | 6,668 |
| 0.1 | 7,455 | 12,900 |
| 0.5 | 37,275 | 58,640 |
| 1.0 | 74,550 | 111,900 |
Ion concentration and conductance
Ions have different capacities to convey electricity. For example, calcium ions convey more electrical current per equivalent than sodium ions, and potassium ions have a greater capacity to convey current than calcium ions. Water samples with identical total concentrations of salts can therefore have somewhat different specific conductances because concentrations of individual ions often differ among samples.
These discrepancies are not a major factor in considerations of specific conductance and salinity relationships in seawater or estuarine water, for their ionic concentrations are relatively constant in proportion. In fact, some conductivity meters are calibrated to measure both specific conductance and salinity. The salinity measurement is based on extrapolation of salinity from the normal relationship between specific conductance and salinity in seawater.
Seawater-specific conductance
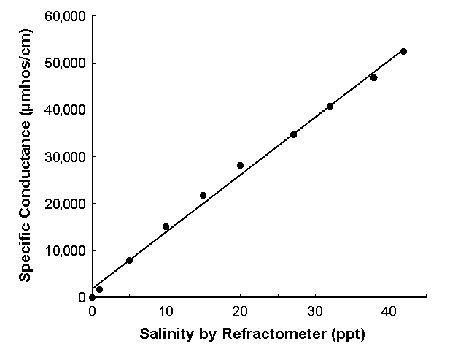
Normal seawater usually has a specific conductance of around 50,000 µmhos per centimeter. Its conductance increases with salinity, as shown in Fig. 2.
Dissolved solids and salinity
Dissolved salts usually comprise over 95 percent of the weight of dissolved substances in natural waters, so total dissolved solids concentration and salinity should be approximately equal. In seawater, total dissolved solids cannot be measured accurately because it is difficult to remove the water of hydration from the residual salts left after evaporation. In waters of 5 ppt salinity and less, however, total dissolved solids can be measured accurately, and the concentration of total dissolved solids is approximately the same as salinity.
Dissolved solids and conductance
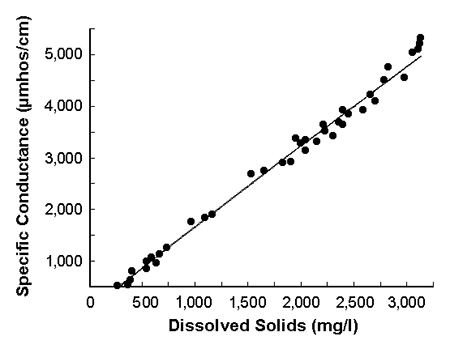
There is a close relationship between specific conductance and total dissolved solids in samples of similar ionic proportions. This relationship allows meters to be calibrated to read total dissolved solids concentrations directly.
These conductivity meters are modified to display total dissolved solids instead of specific conductance. Specific conductance is converted to total dissolved solids concentration using a factor derived from the measured relationship between the two variables. This relationship is illustrated in Fig. 3 for water from the Gila River in Arizona, USA.
Unfortunately, for inland surface waters and ground waters, ionic proportions often vary greatly among locations. The factor for converting specific conductance to total dissolved solids concentration varies among waters from different sources. Because of this, total dissolved solids meters do not provide results that can be compared among locations.
Temperature and conductance
The specific conductance of a water sample will decrease with decreasing temperature. Values can be adjusted to 25 degrees C by dividing the measured conductance by the factor 1 + [0.0191 water temperature – 25 degrees-C].
A sample with a specific conductance of 1,2103 µmhos per centimeter at 22 degrees-C would have a specific conductance of 1,276 µmhos per centimeter at 25 degrees-C. Some conductivity meters have temperature compensators, but if your meter does not have one, failure to correct for temperature can cause small discrepancies in readings.
Reference points
Where conductivity meters are used to estimate the salt content of water, it is best to measure specific conductance directly and not attempt to convert the values to salinity or total dissolved solids. Some points for specific conductance:
- Distilled water usually has specific conductance of less than 2 µmhos per centimeter.
- In humid regions, inland surface waters seldom have specific conductance above 500 µmhos per centimeter.
- In semi-arid regions, specific conductance in inland surface waters can reach 4,000-5,000 µmhos per centimeter, while in arid regions, surface waters can have values above 5,000 µmhos per centimeter.
- Drinking water has specific conductance of 50-1,500 µmhos per centimeter.
- Inland shrimp production usually is done in waters of 2-5 ppt. This salinity range usually corresponds to specific conductance of 2,500-7,000 µmhos per centimeter.
- Seawater has an average specific conductance of around 50,000 µmhos per centimeter.
- A multiplier of 0.0006 is recommended for converting specific conductance (in mhos per centimeter) to salinity (in ppt) for inland ground and surface waters, while a multiplier of 0.0007 is recommended for brackish water and seawater.
Additional conductance measurement
A second method can also be used to report specific conductance values. Some conductivity meters display specific conductance in millisiemens per meter (mS per meter) according to the International System of Units. These values can be converted to µmhos per centimeter by the following relationship: 1 mS per meter = 10 mhos per centimeter and 1 mS per centimeter = 1 µmho per centimeter. Thus, multiply mS per meter by 10 to obtain µmhos per centimeter.
Conclusion
With activities like shrimp farming in low-salinity water becoming more common, more accurate ways of measuring low salinity are needed. Measurement of specific conductance is a valid alternative to salinity measurement by refractometry.
(Editor’s Note: This article was originally published in the February 2002 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Author
-

Claude E. Boyd, Ph.D.
Professor, Department of Fisheries and Allied Aquacultures
International Center for Aquaculture and Aquatic Environments
Auburn University
Auburn, AL 36849 USA
Tagged With
Related Posts

Responsibility
Accuracy of custom water analyses varies
The reliability of trace element analyses reported by custom laboratories cannot be checked by simple techniques, and results may not always be accurate. One should check the reliability of major ion analyses by determining the charge balance and comparing the measured total ion concentration with the total ion concentration estimated from conductivity.

Responsibility
Defining ranges for water quality variables presents complex, challenging process
The creation and application of reference tables for acceptable concentration ranges of physical and chemical water quality variables for culture organisms would be challenging due to the differing tolerances found among the many farmed species.

Responsibility
Electrical conductivity of water, part 1
The electrical conductivity of water, usually called specific conductance or simply conductivity, is an important property of water frequently measured in aquaculture systems, and provides an assessment of the total concentration of dissolved ions in water.

Responsibility
Electrical conductivity of water, part 2
The electrical conductivity of water is frequently measured in aquaculture systems and taken as an indicator of the degree of mineralization (total ion concentration) of water.

