Studies conducted at the National Center for Mariculture, Israel

A number of studies have reported that dietary arachidonic acid (ArA, 20:4 omega-6) improves fish survival as well as growth, provided it is offered together with suitable levels of the other essential fatty acids eicosapentaenoic acid (20:5 omegan-3) and, in particular, docosahexaenoic acid (22:6 omega-3).
Researchers at the National Center for Mariculture in Eilat, Israel, have carried out a series of studies that varied dietary levels of ArA fed to larvae of gilt-head sea bream (Sparus aurata) with the aim to improve their survival following exposure to various types of stress.
Improved survival after stress
The initial studies showed dietary ArA markedly improved larvae survival following the acute stress of tank transfer. Moreover, feeding ArA prior to handling was much more effective than feeding this fatty acid following the stress event (Fig. 1).
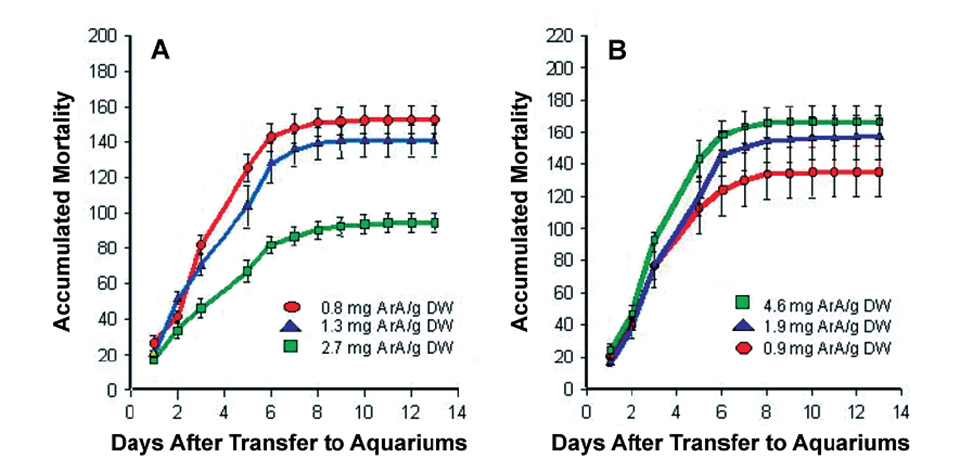
A – ArA levels in rotifers fed prior to handling stress
B – ArA levels in artemia fed after handling stress.
In a further trial, the authors found that different ages of sea bream larvae raised on artemia with high ArA levels and subjected to the acute stress of air exposure for 90 seconds clearly exhibited reduced cortisol levels compared to larvae fed low dietary ArA (Fig. 2).
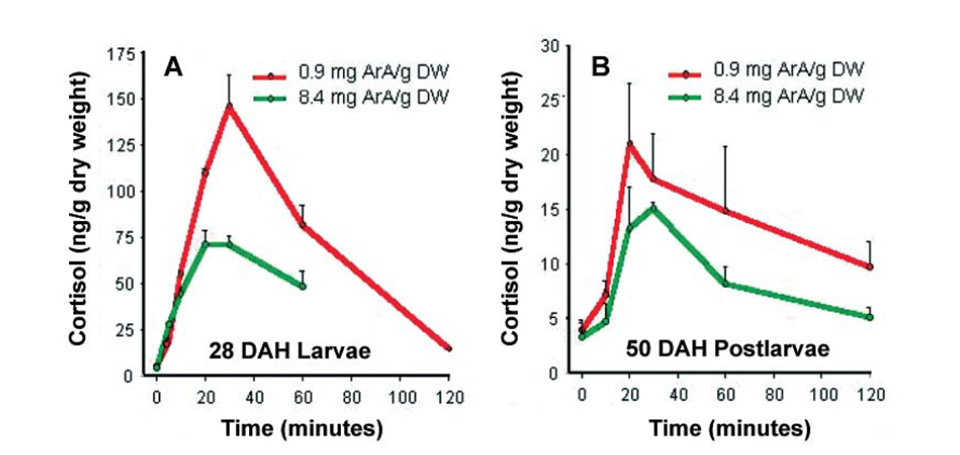
A – larvae and B – postlarvae of gilthead sea bream fed Artemia ArA treatments.
Decreased cortisol synthesis during handling stress
Dietary ArA is readily incorporated into cell membrane phospholipids and released by an activated membrane phospholipase, primarily PLA2. This release is immediately followed by enzymatic conversion of ArA into a broad range of highly biologically active metabolites, collectively known as eicosanoids. The conversion occurs via cyclooxygenase pathways into prostaglandins and thromboxanes, into leukotrienes and hydroxyeicosatetraenoic acids by lipoxygenase, and into epoxyeicosatraenoic acids by epoxygenase.
These paracrine and/or endocrine hormones participate in cellular regulation, hormone release, osmoregulation, and organ function in fish. Prostaglandins derived from ArA (PGE2) have been shown to modulate the sensitivity of the hypothalamus-pituitary-adrenal axis in mammals, which is responsible for the release of cortisol in response to stress.
The authors reasoned that PGE2 in fish down regulated or decreased cortisol synthesis through the homologous hypothalamus-pituitary-interrenal axis by changing the sensitivity of the interrenal cells to the hormone adrenocorticotropic hormone, which signals the release of cortisol in a dose-dependent manner. The down regulation of cortisol appears to have given a survival advantage to larvae fed elevated ArA levels and then subjected to acute handling stress.
Apart from being involved in the catabolism of energy-yielding substrates, cortisol contributes to restoring the hydromineral balance, which is impaired by the earlier release of catecholamines during the stress response. In addition, cortisol reduces the immune response, preventing detrimental inflammation.
Overall, the release of cortisol can be considered an adaptive mechanism to enhance survival by enabling a strong energy-demanding response while maintaining homeostasis. This is provided cortisol levels do not remain high and quickly return to basal levels during recovery from the stressor.
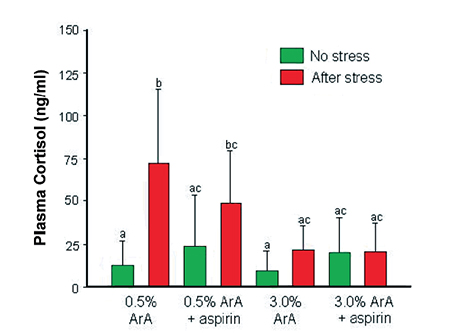
The response of adult sea bream previously fed pellets containing low ArA at 0.5 percent of total fatty acids or high ArA at 3.0 percent of total fatty acids to five minutes of net confinement also showed that high dietary ArA down regulated cortisol levels when fish were exposed to this type of acute stress (Fig. 3).
On the other hand, when aspirin, a known inhibitor of prostaglandin synthesis, was added to the treatment pellets, down regulation of cortisol still occurred and was even more pronounced in the high-ArA diet. This suggested that ArA reduced cortisol levels not as PGE2 but as free ArA or some other ArA-derived eicosanoid.
Increased cortisol synthesis during chronics salinity stress
In another recent study at the National Center for Mariculture, premetamorphosing and metamorphosing sea bream larvae fed different levels of ArA were exposed to handling stress or both handling stress and chronic daily salinity fluctuation (25 to 40 ppt) for 10 days. At the end of the trial, there was a clear correlation in both age classes between the dietary level of ArA and survival in fish exposed only to acute handling, which reinforced previous findings (Fig. 4). On the other hand, fish in both age classes exposed to chronic salinity stress showed a marked decrease in survival at the highest dietary ArA level, which was particularly pronounced in the premetamorphosing larvae.
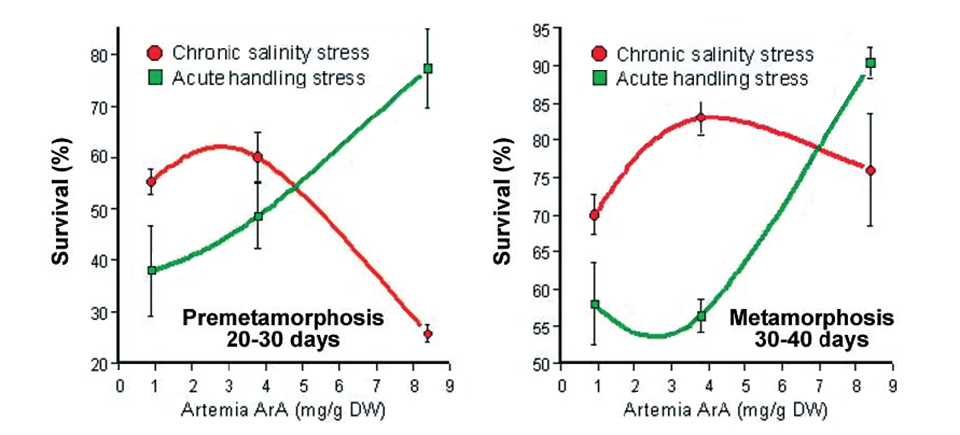
Basal cortisol levels of the metamorphosing postlarvae at the end of the experiment demonstrated significant differences as a function of stress type and dietary ArA. In fish exposed only to the acute stress of handling, basal body cortisol was independent of dietary ArA. Larvae exposed to daily salinity change, however, demonstrated distinctly higher basal cortisol levels that significantly increased with rising ArA levels in the artemia they consumed.
These findings suggested the effect of dietary ArA on cortisol production may be intimately linked not only to the type of stress but also the duration of stress exposure. It is important to note that sustained high levels of circulating cortisol can severely compromise the effectiveness of the immune system and lead to disease and high mortality. This may have been the predominant factor causing the high mortality observed in the salinity-stressed larvae fed elevated levels of ArA.
Possible links: ArA, stress type, cortisol synthesis
The mechanisms that link dietary ArA, stress type, and cortisol synthesis may occur within the interrenal cell. ACTH stimulates cortisol synthesis in a dose-dependent manner when binding to its receptors on the interrenal cell membrane. This initiates a signaling cascade integrating G-proteins, adenyl cyclase, cyclic adenosine mono-phosphate, and protein kinases that relay the hormonal signal to the cellular machinery in the mitochondria to synthesize cortisol.
A plausible scenario is that when ArA from the interrenal membrane is released and converted to prostaglandins, leukotrienes, and free ArA, these metabolites act as second messengers to modulate the signaling cascade to increase or decrease cortisol synthesis. A number of authors have said interrenal PGE2 might inhibit ACTH binding to the membrane, which would reduce cortisol synthesis. Alternatively, leukotrienes may stimulate ACTH production to up regulate cortisol.
Other studies have reported that intracellular free arachidonic acid, as a second messenger, appears to modulate protein kinase C to up regulate cortisol synthesis. Conversely, as levels of intracellular free ArA increase, they can have the opposite affect and modulate protein kinase to down regulate cortisol synthesis.
Clearly, the net effect of different intracellular levels of free ArA, prost-glandins, and leukotrienes determines how cortisol synthesis is regulated in the interrenal cell. However, how acute or chronic stress determines the intracellular levels of ArA and its metabolites in the interrenal cells requires a great deal more work.
Conclusion
Studies at the National Center for Mariculture suggested that stress response in fish is under stronger dietary control than previously thought. It appears that mortality associated with acute stressors in fish culture can be decreased through dietary manipulation of arachidonic acid in the presence of suitable levels of eicosapentaenoic acid and docosahexaenoic acid.
On the other hand, the dietary requirements for these essential fatty acids will likely be specific to species and developmental stage. Moreover, inappropriate levels of dietary ArA may further reduce survival in larvae exposed to chronic stressors from the environment or poor farm management.
(Editor’s Note: This article was originally published in the October 2003 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
William Koven, Ph.D.
Israel Oceanographic and Limnological Research
The National Center for Mariculture
P.O. Box 1212
Eilat, Israel -
Amos Tandler, Ph.D.
Israel Oceanographic and Limnological Research
The National Center for Mariculture
P.O. Box 1212
Eilat, Israel -
Rogier van Anholt, M.Sc.
Department of Animal Ecology and Ecophysiology
University of Nijmegen
Nijmegen, The Netherlands
Tagged With
Related Posts

Health & Welfare
Advances in fish hatchery management
Advances in fish hatchery management – particularly in the areas of brood management and induced spawning – have helped establish aquaculture for multiple species.

Aquafeeds
Aquaculture Exchange: Giovanni Turchini, Deakin University, part 2
In the second part of our interview with the associate head of research at the School of Life and Environmental Sciences at Deakin University (Australia) he discusses the various alternative sources of omega-3 fatty acids coming on to the market and why research is crucial to advancing aquaculture.

Aquafeeds
Aquafeed moonshots at the F3 ‘talent show’
At the F3 (fish-free feed) Companies Got Talent event in Burlingame, Calif., last week, alternative (non-marine) aquafeed ingredient companies spoke of decoupling aquaculture from fishmeal and fish oil in their quest for greater sustainability.

Health & Welfare
Children’s hospital Boston makes zebrafish feeding regime breakthrough
Zebrafish offer a living model system for research in a wide variety of scientific disciplines. The Aquatic Resources Program at Children’s Hospital Boston recently identified an alternative feeding method that requires less labor.


