Low numbers can be detected from fish tissue using PCR

Columnaris disease is a significant problem in commercially produced channel catfish (Ictalurus punctatus). Annual monetary losses attributed to this pathogen are second only to those due to “hole-in-the-head disease,” enteric septicemia of catfish. The causative agent of columnaris disease is Flavobacterium columnare, a gram-negative, long rod-shaped bacteria that is ubiquitous in freshwater environments. Typically this bacterium causes a saddle-back lesion in channel catfish.
Reproducible challenge model needed
Significant time and money have been spent on developing means to treat or control columnaris disease, but these efforts have been greatly hindered by the lack of a readily reproducible bacterial challenge model that would allow scientists to compare results from test to test and lab to lab. Such a model is a prerequisite for the establishment of efficacy and eventual governmental approval of any therapeutic agent.
Experimental trials
To help establish a model, recent trials by the authors at the U.S. Department of Agriculture Agricultural Research Service’s Aquatic Animal Health Research Unit in Auburn, Alabama, USA, evaluated the effects of several treatments on pathogen uptake and the susceptibility of fish exposed to columnaris disease.
Abrasion and bacterial immersion
Previous studies by the authors demonstrated that low numbers of F. columnare cells can be detected directly from fish tissue using polymerase chain reaction (PCR). In a series of experiments, PCR was initially used to access the ability of the F. columnare cells to enter the host fish following abrasion and subsequent bath immersion in the bacteria. Combined PCR and challenge studies evaluated four trials – abraded and bacterially challenged, unabraded and bacterially challenged, abraded and sham challenged, and normal controls – with three replicates each.
Three fish from a pool of 30 fish per treatment were sampled five times following either bacterial challenge or sham challenge. Abrasion involved first sedating the fish in a separate tank and then physically abrading each fish using a sterile dry gauze. This cloth was dragged twice, back and forth, along the lateral line on the left lateral surface of each fish. Sham challenges involved immersion of fish in sterile bacterial growth broth.
Mortality following challenge, recorded daily for two weeks after the PCR sampling portion of the study, was used to determine cumulative percent survival. During the study, three fish were sacrificed at zero, five, 15, 30 and 60 minutes following bacterial or sham challenge. Samples of mucus, skin, blood gill, trunk kidney, and liver were collected using aseptic techniques, and DNA was extracted. These template DNA samples were analyzed using nested species-specific PCR for the presence of F. columnare.
Abrasion methods tested
In another series of experiments, various techniques to disrupt the epithelium were evaluated for their ability to predispose channel catfish to columnaris diseases. Methods evaluated were physical abrasion, acid burn, hot branding, and cold branding. All methods were administered to the left and right side of the fish.
Cold branding involved a wire placed on dry ice for 20 seconds, while heat cautery used a wire placed over a Bunsen burner for 20 seconds. Acid burns involved applying 0.1-Mol ammonium hydroxide via a paint brush.
The four treatments and one unabraded control tank of 20 fish each were challenged by immersion exposure for 60 minutes in 75 l of water with 1 x 108 colony-forming units per milliliter of 24-hour-old F. columnare grown at 29 ± 2 degrees-C. Each challenged tank was observed for mortality for two weeks following challenge.
Histopathology
In a final series of experiments, 20 catfish were abraded and challenged using the above methods and monitored for mortality for four days following challenge. Histopathology samples with five fish per time point were concurrently taken from each group at four, 24, 48, 72 and 96 hours. These samples were stored in neutral buffered formalin, embedded in paraffin, and stained with hematoxylin and eosin.
Slime replacement
As part of the previous series of experiments, a group of catfish was treated with an aloe vera-derived “slime replacement” compound, following the pretreatment. This group was evaluated to determine if the effects of the pretreatment could be mitigated by coating the host skin.
Results
The universal 16S rRNA gene primer set yielded the expected 1,500 bp product for that gene, while the F. columnare species-specific 16S rRNA gene primer set, FvpF1-FvpR1, yielded the expected 1,193 bp product. Detection of the bacterium within DNA templates derived from external tissue sources was possible as early as five minutes after challenge and could be detected up to the one-hour cutoff of the experiment.
The uptake of bacteria differed significantly between abraded and unabraded fish at five minutes, but not at one hour. Mucus, skin, and gill were the most reliable tissues for the early detection of F. columnare.
Bacterial detection in liver took 60 minutes in unabraded fish, but only 30 minutes in abraded fish. Conversely, bacteria in kidney were detected after only five minutes in unabraded fish, but not until 60 minutes in abraded fish. Blood was the only tissue where abrasion had a significant positive effect on detection time, with bacteria detailed after five minutes in abraded fish and 60 minutes in unabraded fish. Abrasion in all other tissues did not significantly alter bacterial detection. The cumulative percent survival for the abrasion challenge with F. columnare was significantly less for abraded fish.

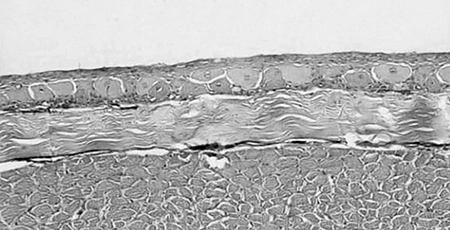
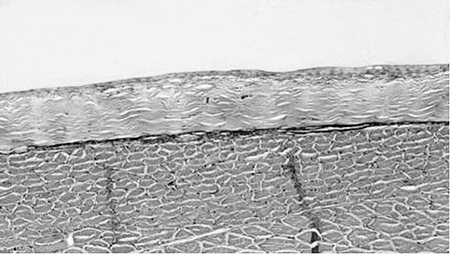
When compared to control fish samples (Fig. 1), histopathology samples taken over time from catfish with physical abrasion, acid burn, hot branding, and cold branding showed significant changes to underlying epithelial tissue and musculature (Figs. 2 and 3). The greatest change was observed in catfish that were physically abraded and those that were cauterized. The epithelial surface of the abraded fish showed some regeneration at 24 hours after treatment and was almost completely regenerated in two days.
All treatments increased catfish susceptibility to columnaris disease in the bacterial challenge portion of the study. A cumulative percent survival of 40 to 60 percent was observed, while no mortality was recorded in the control catfish.
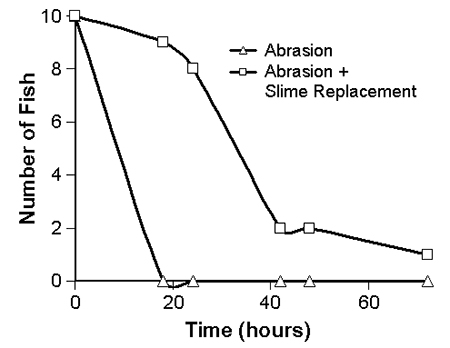
The abrasion and heat cautery treatments produced significantly higher mortality than the other two treatments. This mortality decreased as the interval between scarification and challenge increased up to three days. Catfish that were abraded or cauterized and treated with slime replacement before challenge showed a delay of death of almost two days compared to those not treated (Fig. 4).
Conclusion
Recent increases in cases of columnaris indicate the channel catfish industry is loosing its fight against the disease. To win the fight, it must determine the conditions under which columnaris occurs, which will then allow advances in the control of the pathogen. For instance, such advances will allow more rapid testing of live bacterin vaccines under development at the U.S. Department of Agriculture Agricultural Research Service’s Aquatic Animal Health Research Unit.
For some time, its work and that of others indicated that the presence of F. columnare alone was not sufficient to cause clinical disease. Lab results demonstrated that both abraded and unabraded catfish acquired F. columnare in all tissues tested within an hour of challenge. Yet unabraded fish had no evidence of clinical disease, whereas abraded fish exhibited the typical saddle-back lesions and died.
In a series of experiments that compared models of disease causation, disruption of the epithelium appeared to be the major prerequisite for disease and could be reversed rather simply by the application of an artificial slime agent. The authors found the best methods of predisposing fish to columnaris disease were either physical abrasion or cautery branding. Both methods showed regeneration after two days, indicating rapid epithelial disruption was more important than long-term disruption or method.
In work yet to be published, the authors also observed that when comparing models of injury that might occur due to handling or deteriorating water conditions, one feature of infection was clear: The larger the wound or abrasion, the more bacteria are able to enter the fish and cause disease. However, protection of wounds with artificial slime or a reduction in the dose or number of F. columnare in the water significantly lowers columnaris incidence in fish. These findings offer significant promise in the control of columnaris following the harvest or shipping of fish.
(Editor’s Note: This article was originally published in the August 2003 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Joel A. Bader, Ph.D.
United States Department of Agriculture
Agricultural Research Service
Aquatic Animal Health Research Laboratory
P.O. Box 0952
Auburn, Alabama 36831-0952 USA -
Kenneth E. Nusbaum, DVM, Ph.D.
Department of Pathobiology
College of Veterinary Medicine
Auburn University
Auburn, Alabama, USA
Related Posts

Health & Welfare
Antigens provide immunity against ich in channel catfish trials
Vaccination against the Ich parasite is an alternative to chemical treatment. Fish develop a humoral immune response to trophont antigens, with the degree of protection related to the immunizing doses of trophonts used.

Innovation & Investment
Assessing coloration in channel catfish fillets
Because consumers look at color to gauge quality of catfish fillets, the authors developed a digital photography measurement method to assess yellowness.

Health & Welfare
Blue catfish outproduce channel catfish under low-D.O. conditions
Although there is increasing interest in blue catfish, a potential disadvantage of the fish when compared to channel catfish is their reported poorer tolerance of low dissolved-oxygen concentrations.

Health & Welfare
CART regulates food intake in channel catfish
Experiments by the authors showed that the gene activity of the brain neurotransmitter CART changed in catfish in response to feed intake.


