Sequence-specific gene silencing part of functional genomics, targeted gene therapies
Shrimp aquaculture represents one of the fastest-growing segments of aquaculture with annual production in excess of 2.5 million metric tons (MT) and a farm-gate value of U.S. $14 billion to $16 billion. However, a major obstacle to the continued growth of shrimp aquaculture is the emergence of viral diseases. Collectively, it is estimated that disease agents have reduced the production capacity of the industry by as much as 40 percent with annual losses in excess of $3 billion.
Current strategies to prevent viral disease include the use of specific pathogen-free and specific pathogen-resistant stocks of shrimp, immune stimulation, quarantine and environmental management. Together, these measures have reduced the impacts of viral diseases, but there continues to be a critical need for more effective methods of controlling viral infections.
RNA interference
Therapeutics based on RNA interference (RNAi) have tremendous potential to block viral disease progression by silencing genes essential for viral replication or pathogenicity. RNAi is a cellular mechanism dependent on double-stranded RNA (dsRNA) conserved from plants to humans that is thought to have evolved as a component of the host’s immune response to viruses and transposable elements. The RNAi machinery, which allows sequence-specific gene silencing, has now emerged as a key target for multiple applications ranging from functional genomics to targeted gene therapies.
RNAi protection in shrimp
Research has confirmed the presence of an active RNAi response in shrimp, and dsRNA matching essential genes of important shrimp viral pathogens such as white spot syndrome virus (WSSV), Taura syndrome virus and yellow head virus delivered by injection has yielded high levels of protection.
The authors’ initial laboratory efforts on RNAi focused on characterizing the RNAi response in Pacific white shrimp (Litopenaeus vannamei) using WSSV challenge models (Fig. 1). Results indicated that 80 to 90 percent levels of WSSV-specific protection could be achieved with no apparent off-target effects at dosage levels as low as 200 ng dsRNA/gram shrimp. Significant levels of protection were observed up to 26 days after the injection of a single dsRNA dose. In addition, dsRNA was produced using in-vitro/synthetic methods or bacterial expression systems.
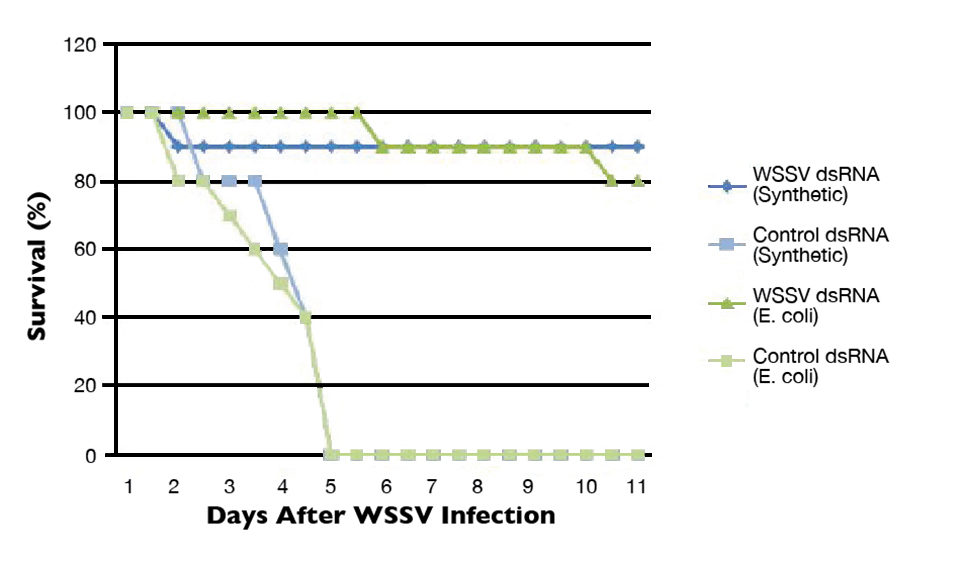
The ability to use bacterial expression systems to produce dsRNA is particularly promising in aquaculture applications, as it potentially provides a more cost-effective method of producing RNAi-based therapeutics. Likewise, the persistence of RNAi-based protection over time suggests that dsRNA is energetically stable and remains intact in the cells or hemolymph. RNAi-based therapeutics could therefore have a reasonably long-lasting protective effect in shrimp, and may not require continuous daily applications.
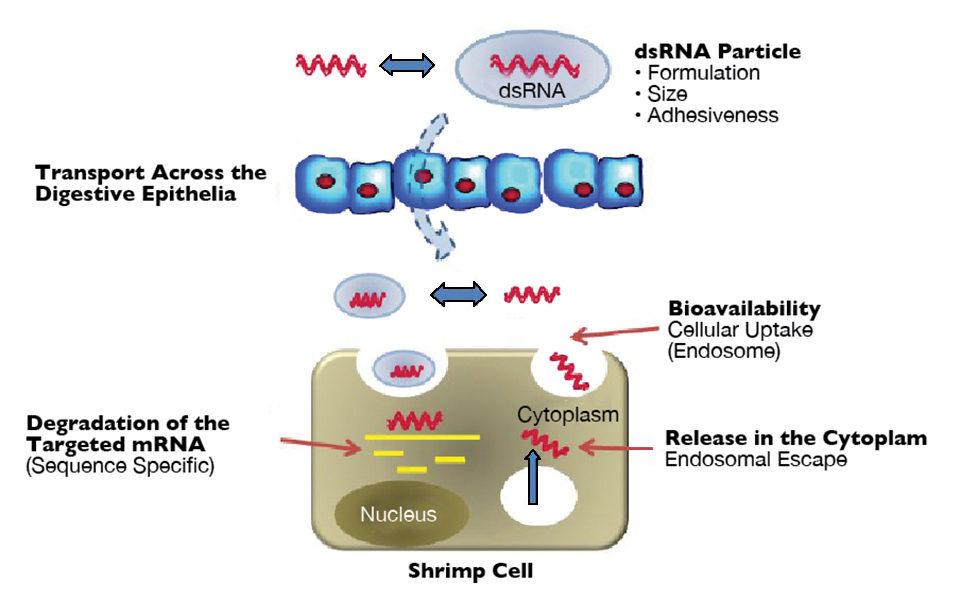
Oral delivery of RNAi therapeutics
Unfortunately, the delivery of potential RNAi-based therapeutics via injection is impractical. Oral delivery approaches are likely to be a more viable option in shrimp aquaculture. To date, simple methods for the oral delivery of dsRNA to Pacific white shrimp have not proven successful and reproducible.
Trials conducted by the authors at Aqua Bounty Technologies have included varied evaluations of feeds coated with or incorporating synthetic or bacterially expressed dsRNA, the use of protein-based additives such as histones and polylysines to facilitate uptake, and artemia-mediated approaches to orally deliver dsRNA.
These studies have not yielded protective trends in repeated studies, which may be attributable to varying degrees of degradation of the dsRNA in the food, water or guts of the shrimp; insufficient dsRNA delivered across the shrimp guts or differential uptake of the dsRNA. Thus, a consistent method for effective stabilization, delivery and transport of bioactive dsRNA remains needed to make oral delivery of an RNAi-based therapeutic feasible.
Oral delivery evaluation
In-vivo evaluations of oral RNAi therapeutics can be conducted using dsRNA in conjunction with a pathogen challenge model. However, these assays are resource-intensive and difficult to conduct reliably, and information on the partial efficacy of oral formulations can be difficult to detect.
To address this problem, the authors sought to develop an alternative approach for evaluating the oral delivery of dsRNA by incorporating the use of dsRNA products targeting endogenous host genes. Host gene-silencing models have several potential advantages for use in screening studies, including the increased ability to detect gene silencing at low dsRNA doses, improved gene copy quantification capabilities that facilitate treatment comparisons and better assay consistency, which allows comparisons of more treatment groups using fewer shrimp.
Injection
To identify suitable endogenous gene targets for a screening model, the authors initially cloned L. vannamei clotting protein, prophenoloxidase and pigment-dispersing hormone (PDH I and II) genes. Corresponding dsRNAs were injected, and animals were assessed for RNAi-based changes in phenotypes.
Injection of dsRNA specific to clotting protein resulted in the absence of spontaneous hemolymph coagulation, while hemolymph from controls injected with non-specific dsRNA consistently formed a very stable clot. Injection of prophenoloxidase -specific dsRNA resulted in a marked decrease in hemolymph melanization, while non-specific dsRNA had no effect on melanization. Injection of PDH-specific dsRNA resulted in a marked decrease in pigment-dispersing capacity following transfers between holding tanks with different background colors.
Dose response studies indicated that 300 ng, 500 ng and 1 mg of dsRNA were sufficient respectively to impair clotting, melanization and pigment dispersion. Based on these results, clotting protein was selected for further model development and characterization.
Injection studies conducted with clotting protein dsRNA yielded significant silencing in all tissues tested, leading up to a 1,000-fold reduction in targeted transcript relative to control tissue specific transcript levels (Fig. 2).
Results from additional injection studies with varied quantities of clotting protein dsRNA indicated that doses between about 2.5 and 25 ng were sufficient for the detection of gene silencing, and the response remained fairly consistent over at least 15 days.
These results indicated that the ability to detect gene silencing increased 10- to 100-fold compared to detection limits using in-vivo WSSV challenge models. With screening models such as the bioassays for endogenous gene silencing described here, high-throughput evaluation of multiple formulations for oral delivery of dsRNA becomes practical and will be a powerful method for empirical evaluations of dsRNA-packaging technologies.
Perspectives
The potential of RNAi-based therapeutics for use in targeting viral shrimp pathogens remains high, given compelling advantages including high levels of protection compared to other available treatments, a defined mode of action and relatively low effective dosing. dsRNA is able to cross tissues and cell membranes, and trigger a systemic RNAi response that remains effective over several weeks.
Cost-effective bacterial expression systems can be used to produce dsRNA. In addition, dsRNA can be designed to silence single or multiple viral genes, allowing specific protection against multiple pathogens. New dsRNA sequences can be rapidly tested to address emerging viral pathogens.
The lack of a relatively inexpensive, safe and efficacious oral delivery system remains a critical challenge to the successful development of this class of therapeutics. Screening models based on endogenous shrimp genes provide useful tools that improve our ability to quickly and accurately assess the stability and bioavailability of orally delivered dsRNA formulations.
Potential approaches to be tested with this model include encapsulation technologies, yeast- or algae-based expression and delivery systems, and varied factors that alter digestion characteristics or improve packaging and dsRNA uptake.
(Editor’s Note: This article was originally published in the July/August 2010 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Xavier Lauth, Ph.D.
Senior Research Scientist
Aqua Bounty Technologies
8395 Camino Santa Fe, Suite E
San Diego, California 92121 USA[109,111,99,46,121,116,110,117,111,98,97,117,113,97,64,104,116,117,97,108,120]
-
Jason Stannard
Aqua Bounty Technologies
8395 Camino Santa Fe, Suite E
San Diego, California 92121 USA -
John Buchanan, Ph.D.
Aqua Bounty Technologies
8395 Camino Santa Fe, Suite E
San Diego, California 92121 USA
Tagged With
Related Posts

Health & Welfare
A comprehensive look at the Proficiency Test for farmed shrimp
The University of Arizona Aquaculture Pathology Laboratory has carried out the Proficiency Test (PT) since 2005, with 300-plus diagnostic laboratories participating while improving their capabilities in the diagnosis of several shrimp pathogens.

Health & Welfare
Developmental transcriptomes from penaeid shrimp
To develop an alternative mechanism for genetic copyright of improved shrimp lines, the authors used a bioinformatics approach to identify germ line genes that could be potentially targeted to ablate the germ line.

Innovation & Investment
AquaGen CEO: Genomics are transforming aquaculture
The CEO of AquaGen knew that the Norwegian research group’s work in genomics was key to the salmon industry’s future. And that was before she even worked there.

Health & Welfare
Finfish genetic improvement
Although the application of selective breeding and genetics can yield dramatic results, the use of genetically improved stock varies widely among aquaculture sectors. Virtually all Atlantic salmon and rainbow trout producers use improved stock, while use of genetically improved tilapia varies widely.


