Acid-resistant PLGA can protect vaccine through GI tract
Infectious hematopoietic necrosis virus (IHNV) is the most significant viral pathogen for rainbow trout in the United States. It causes millions of dollars of losses in the U.S. state of Idaho, where over half of the country’s rainbow trout are farmed.
Protecting fish from infections is an effective strategy against viral disease in aquaculture, and vaccination plays an important role in disease prevention. In the United States, the current vaccination method for rainbow trout against IHNV, immersion of the fish with a killed vaccine, does not provide sufficient exposure to the pathogen for adequate absorption and lifelong immunity.
In Canada, a DNA vaccine for IHNV has been licensed for use in Atlantic salmon (Salmo salar). This vaccine is effective against the virus, but has not been seriously considered for use in the U.S. rainbow trout industry because the required intramuscular injection is cost prohibitive.
Oral delivery vaccine
The author’s research teams at Idaho State University, University of Idaho and Clear Springs Foods, Inc. are currently working on a system for oral delivery of IHNV DNA vaccine via fish feed that would provide a more practical vaccine delivery option. The goal of this research is to adapt the proven DNA vaccine for oral delivery to rainbow trout through encapsulation with either a polymer – poly (D, L-lactide co-glycolide), PLGA – or liposome with subsequent incorporation in fish feed.
Trials with rainbow trout (Oncorhynchus mykiss) and Atlantic salmon have shown that PLGA can potentially be used as an oral delivery vehicle. PLGA is acid resistant and thus can protect the vaccine through the gastrointestinal tract, allowing it to reach the hindgut of the fish.
Additionally, this copolymer is versatile in that it can be formulated using various portions of lactide and glycolide, which determine the rate of release of the vaccine under given conditions of acidity. Utilizing these polymer/vaccine particles allows the treatment to either lodge in the mucosal membranes of the fish or cross the membranes and enter the bloodstream, thus allowing sustained release of DNA plasmid that can last from hours to days.
Liposome assist
Mammalian research on oral delivery of plasmid DNA vaccines suggests liposomes also can be used to protect plasmid DNA from acidic conditions and enzyme digestion. Liposomes are a mixture of natural phospholipids, phospholipids modified by hydrogenation, synthetic phospholipids and cholesterol. Given their lipophilic properties, liposomes can facilitate the uptake of the DNA vaccine into cells.
Previously, liposomes were used to deliver DNA to rainbow trout by water immersion. However, toxic effects to gill tissues were reported with this method. Delivery of liposomes in feed should help reduce toxic effects by reducing the amount of product that is directly in the water.
Vaccine development
IHNV is a member of the virus family Rhabdoviridae. A detailed analysis of the Western Regional Aquaculture Consortium strain of INHV from southern Idaho revealed that IHNV has six virus genes. Among these genes, surface glycoprotein (G-gene) is the major antigen responsible for the serological properties of the virus.
Using a vector similar to that used to produce the efficacious DNA vaccine in Canada, the authors cloned the G-gene from the Idaho strain of IHNV and grew it in Escherichia coli bacteria for mass production. A solvent evaporation technique then created PLGA nanoparticles with approximately 2 µg DNA/mg PLGA.
In vivo assessment
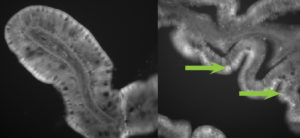
In current studies supported by the Idaho State Board of Education, liposome/DNA complexes were developed by using a thin lipid film hydration method. It was estimated that 30 percent of the initial DNA was entrapped in the complex at a concentration of about 1.5 µg mg-1 of dried liposome.
To assess the in vivo absorption of the PLGA/DNA nanoparticles and liposome/DNA complexes by rainbow trout, a fluorescent dye was included in the formulations instead of DNA to provide a visual marker of tissue particle uptake. The PLGA nanoparticles were either applied directly to a commercial feed diet or resuspended in a buffer for delivery via intubation. The four treatment groups were control, oral feed, oral intubation and anal intubation. Fish tissues were assessed 96 hours after delivery by fluorescent microscopy.
The liposome trials are in the preliminary stages with only a few orally intubated fish in the trials. A small virus challenge trial was also conducted using PLGA/DNA particles.
Results
No PLGA nanoparticles containing dye were detected within any intestinal tissue sections of the control fish. In both trials, approximately 25 to 50 percent of the fish in the remaining three treatment groups had marked nanoparticles in the epithelial cells of their lower intestinal tracts 96 hours after treatment. Liposome/DNA complexes were also seen in the lower intestinal tissues of trout at 48 hours after oral intubation. In addition, fish that received feed with PLGA/DNA particles had 30 percent greater survival than fish that were not vaccinated after virus challenge.
The studies provided evidence for encapsulation and absorption potential of fluorescent nanoparticles and liposomes for fish fed nanoparticles top-coated on feed. This simple method of delivery to fish may prove useful for administering vaccines orally. However, to reduce particle loss, it may be better to incorporate the particles directly into the feed.
The authors are currently working on a larger-scale trial to confirm these initial results and improve the efficiency of the delivery system so that is an economically viable option in the future.
(Editor’s Note: This article was originally published in the March/April 2010 print edition of the Global Aquaculture Advocate.)
Authors
-
Yan Zheng, Ph.D.
Department of Biomedical and Pharmaceutical Sciences
College of Pharmacy
Idaho State University
921 South 8th Avenue, Stop 8334
Pocatello, Idaho 83209-8334 USA -
John Eley, Ph.D.
Department of Biomedical and Pharmaceutical Sciences
College of Pharmacy
Idaho State University
921 South 8th Avenue, Stop 8334
Pocatello, Idaho 83209-8334 USA -
Ram Veerubhotla
Department of Biomedical and Pharmaceutical Sciences
College of Pharmacy
Idaho State University
921 South 8th Avenue, Stop 8334
Pocatello, Idaho 83209-8334 USA -
Sophie St. Hilaire, Ph.D.
Department of Biological Science
College of Art and Science
Idaho State University
Pocatello, Idaho, USA -
Malcolm Shields, Ph.D.
Department of Biological Science
College of Art and Science
Idaho State University
Pocatello, Idaho, USA -
Peter Sheridan, Ph.D.
Department of Biological Science
College of Art and Science
Idaho State University
Pocatello, Idaho, USA -
Ryan Marcum
Department of Biological Science
College of Art and Science
Idaho State University
Pocatello, Idaho, USA -
Marie Adomako
Department of Biological Science
College of Art and Science
Idaho State University
Pocatello, Idaho, USA -
Wendy Sealey, Ph.D.
Hagerman Fish Culture Experiment Station
University of Idaho
Hagerman, Idaho, USA -
Brian Donahower, Ph.D.
Hagerman Fish Culture Experiment Station
University of Idaho
Hagerman, Idaho, USA -
Scott LaPatra, Ph.D.
Clear Springs Foods, Inc.
Buhl, Idaho, USA
Tagged With
Related Posts

Health & Welfare
Algae shows promise as alternative DHA source in rainbow trout diets
A growth trial in Canada evaluated the use of algae biomass to increase the concentration of long-chain polyunsaturated fatty acids in the tissues of rainbow trout.

Aquafeeds
A look at corn distillers dried grains with solubles
Corn distillers dried grains with solubles are an economical source of energy, protein and digestible phosphorus to reduce feed costs and fishmeal usage.

Intelligence
A motive, and a market, for farmed fish in Mexico
Boasting ample areas for aquaculture and a robust domestic demand for seafood – not to mention its close proximity to the U.S. market – a land of opportunity lies in Mexico. Fish farming is primed to meet its potential south of the border.

Aquafeeds
Alternative feed ingredient universe to convene at F3 meeting
What started out as a simple yet ambitious contest to drive innovation in the aquafeed sector has evolved into a fully global competition – and collaboration – amongst ingredient suppliers and feed manufacturers.


