Once fish refuse feed, oral antibiotic therapy will be ineffective
Streptococcus spp. are Gram-positive, nonmotile, catalase-negative cocci that are fermentative in glucose and do not form spores. Streptococcosis, the disease they cause, affects more than 20 species of fish. It causes great economic losses and represents a real danger to warmwater aquaculture, particularly intensively cultured tilapia.
The United States Food and Drug Administration (FDA) has approved the antibacterials oxytetracycline and Romet (a 1:5 combination of ormethoprim and sulfadimethoxine) to combat streptococcosis in aquaculture, but their use is limited to channel catfish and salmonid species. For species like tilapia, expanding FDA approval of existing antibacterials to include additional species may be more promising than seeking approval of new drugs to fight streptococcosis.
One of the requirements in the drug approval process in the United States is efficacy studies. The objectives of a recent study by the authors were to determine the in vitro sensitivity of S. iniae to oxytetracycline and the efficacy of oxytetracycline in controlling mortality in blue tilapia (Tilapia aurea) infected with S. iniae.
Experimental trials
Studies of the minimum inhibitory concentration of oxytetracycline against multiple S. iniae isolates indicated general sensitivity at concentrations of 0.25 to 0.50 milligrams per milliliter. In two trials, oxytetracycline was incorporated into the experimental diet fed to experimentally infected fish.
Fish received oxytetracycline at 25, 50, 75 and 100 mg active ingredient per kilogram body weight per day for 14 days. The positive control treatment consisted of nonmedicated fish challenged with S. iniae, while the negative control treatment consisted of nonchallenged and nonmedicated fish. Each treatment had four tanks with 15 fish in each tank.
Results
No clinical signs or gross pathology were observed in the negative control treatment. Such signs were observed primarily in the challenged, nonmedicated fish and medicated fish that died when challenged.
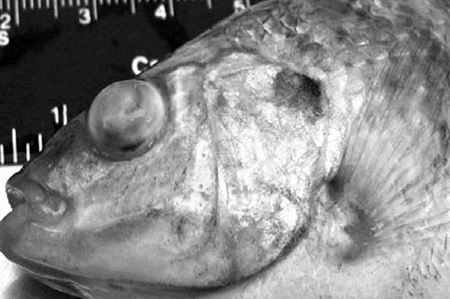
The diseased fish exhibited lethargy and a reduction or cessation of feeding beginning five to six days after infection. The fish swam in circles, in random directions, and on their sides with their bodies arched. Externally, diseased fish had dark skin pigmentation, abdominal distention, hemorrhages, erythema, and eye lesions consisting of bilateral or unilateral exophthalmia, corneal opacity, and complete disintegration of the lens (Fig. 1).
Internally, there was accumulation of abdominal fluid, and the animals’ livers, kidneys, gonads, and gastrointestinal tracts had hemorrhages. The infection was systemic, as shown by the positive isolation of the S. iniae from the brains, kidneys and eyes during the first 22 days postinfection.
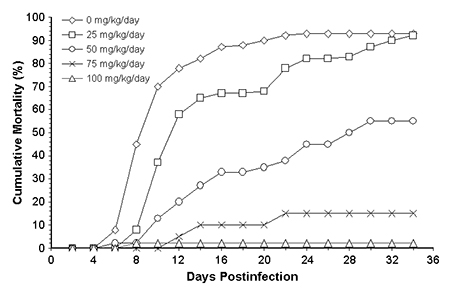
The 50-mg oxytetracycline treatment significantly increased the survival of the infected tilapia from 7 percent in the infected nonmedicated group to 45 percent (Table 1). The 75- and 100-mg oxytetracycline treatments had survival rates of 85 and 98 percent, respectively – significantly higher than the 50-mg treatment (Fig. 2). There was no significant difference between the 75- and 100-mg treatments and the uninfected, nonmedicated treatment.
Darwish, Survival of tilapia infected with S. iniae, Table 1
| Oxytetracycline Dose (mg/kg weight/day) | Survival (%) |
|---|
Oxytetracycline Dose (mg/kg weight/day) | Survival (%) |
|---|---|
| 0 | 7.0 ± 0c |
| 25 | 8.5 ± 1.5c |
| 50 | 45 ± 8.7b |
| 75 | 84.7 ± 5a |
| 100 | 98.2 ± 1.7a |
| Control | 100 ± 0a |
Survivors from the 100-mg oxytetracycline treatment were not found to be carriers of the infection, whereas the bacterium was recovered from 10 percentof the survivors from the 75-mg oxytetracycline treatment.
Conclusion
In trials, oxytetracycline was efficacious in treating S. iniae infection in tilapia, but the reluctance of fish to feed five to six days post-infection emphasizes the critical importance of monitoring and early intervention with oral administration of antibiotics. Once fish start refusing feed, oral antibiotic therapy will be ineffective in reducing fish losses.
Although oxytetracycline appears to be an effective treatment, controlled field trials, target animal safety and tissue residue studies will be required by the FDA to consider its approval.
(Editor’s Note: This article was originally published in the December 2002 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Ahmed M. Darwish, DVM, Ph.D.
Harry K. Dupree Stuttgart National Aquaculture Research Center
United States Department of Agriculture
Agriculture Research Service
P.O. Box 1050
Stuttgart, Arkansas 72160 USA -
Bill R. Griffin, Ph.D.
Harry K. Dupree Stuttgart National Aquaculture Research Center
United States Department of Agriculture
Agriculture Research Service
P.O. Box 1050
Stuttgart, Arkansas 72160 USA
Tagged With
Related Posts

Health & Welfare
Antibiotic-resistant bacteria, part 1
No antimicrobial agent has been developed specifically for aquaculture applications. However, some antibiotic products used to treat humans or land-based animals have been approved for use at aquaculture facilities.

Health & Welfare
Antibiotics in aquaculture: Is responsible use possible?
Regulations on antibiotics in aquaculture vary by country and region, from outright bans to minimal oversight.

Intelligence
Non-compliance on EU antibiotics order may cost India shrimp industry
India, now the world’s top farmed shrimp exporter, may lose access to the European marketplace by 2018 due to ongoing issues with antibiotics.

Health & Welfare
New aquaculture drugs under FDA review
Only eight active pharmaceutical ingredients available in 18 drug products have been approved by the U.S. Food and Drug Administration for use in aquaculture. The approval process can be lengthy and expensive.


