Examining two highly pathogenic viruses in shrimp farming
Viral diseases are the most important cause of shrimp losses in commercial shrimp farming. Taura syndrome virus (TSV) and yellow head virus (YHV) are considered two of the most pathogenic viruses present in shrimp farming in the Western and Eastern Hemispheres, respectively.
YHV and TSV share many of the same target tissues. During the acute phase of infection, TSV and YHV infect the cuticular epithelium of foregut and hindgut, stomach, gills and pleopods. Unlike TSV during the acute phase, YHV also targets other organs, such as the lymphoid organ (L.O.), connective tissue and hemocytes. During the chronic phase of infection, TSV and YHV produce lymphoid organ spheroids in infected shrimp.
Since TSV and YHV share target tissues, and TSV was already present in the Americas at about the time YHV was becoming pandemic in Asia, it is possible that TSV prevented shrimp from becoming infected with YHV during one of the several times in which frozen materials from Asia infected with YHV and white spot syndrome virus resulted in the introduction of only white spot syndrome to shrimp farms in the Americas.
Experimental design
To test this idea, the authors undertook research to evaluate the viral interaction in shrimp preinfected with TSV and then challenged with YHV under experimental conditions. For the work, viral isolates were sourced from the University of Arizona, where TSV isolate obtained from Hawaii, USA, and YHV isolate obtained in 1993 from Thailand had been stored at minus-70 degrees-C.
Two hundred specific pathogen-free Litopenaeus vannamei were stocked in a round 1-m3 tank and challenged with TSV by feeding minced infected tissue at 10 percent of total body weight for one feeding.
For the experimental infection of shrimp preinfected with TSV and then challenged with YHV (TSV-YHV group), three groups were set up. Shrimp in the first group (27DPI) were infected with YHV inoculum 27 days after their exposure to TSV. Those in groups 37DPI and 47DPI received injections of 1.0 x 104 copies of YHV/shrimp in a 100-µL volume 37 and 47 days after TSV exposure.
In a negative control, shrimp were injected with 2 percent saline. Positive control shrimp were infected only with YHV (YHV group).
Ten shrimp were placed in each 90-L tank. Mortality was recorded twice daily from the start of the experiment.
Pleopods from the first abdominal segment were removed from each shrimp to determine the TSV and YHV viral loads. Some samples of the lymphoid organs and adjacent areas were taken to determine the viral loads, as well. For in situ hybridization, histological sections were prepared from fixed and paraffin-embedded shrimp tissues.
Results
Two weeks after exposure to TSV, 28 percent of the infected shrimp survived, went through a typical transition phase and became chronically infected.
The YHV challenge test showed differences in survival among the TSV-YHV groups and shrimp infected only with YHV (Figs. 1 to 3). The YHV groups showed no final survival, while significant differences (P < 0.05) in survival were found among the three groups exposed to TSV prior to challenge with YHV.
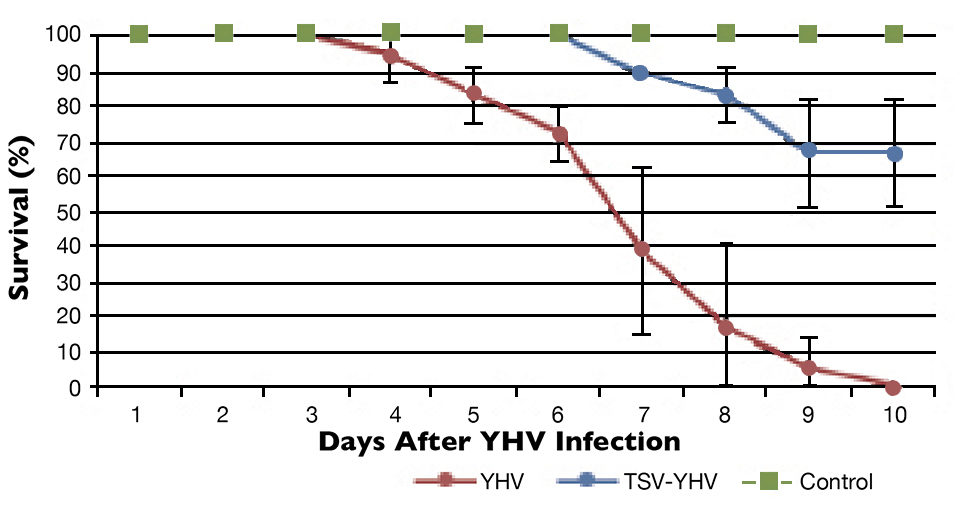
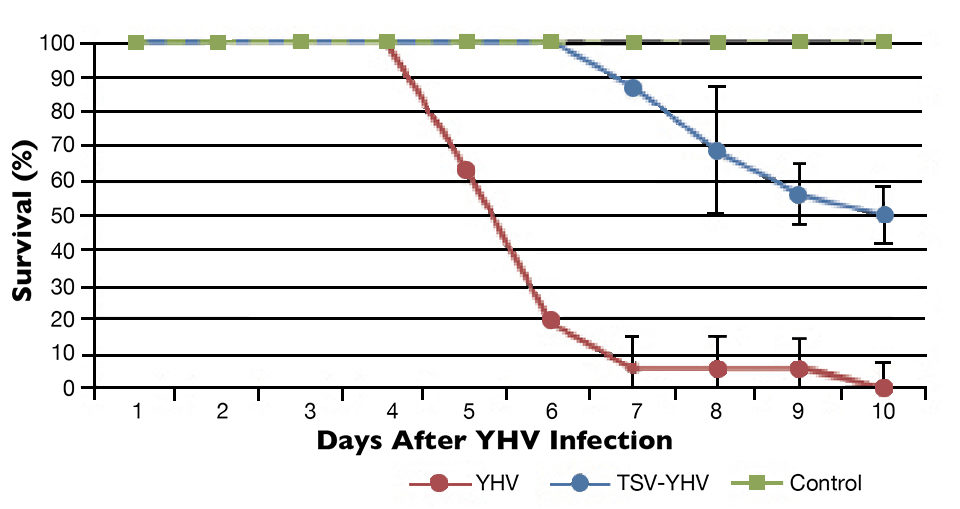
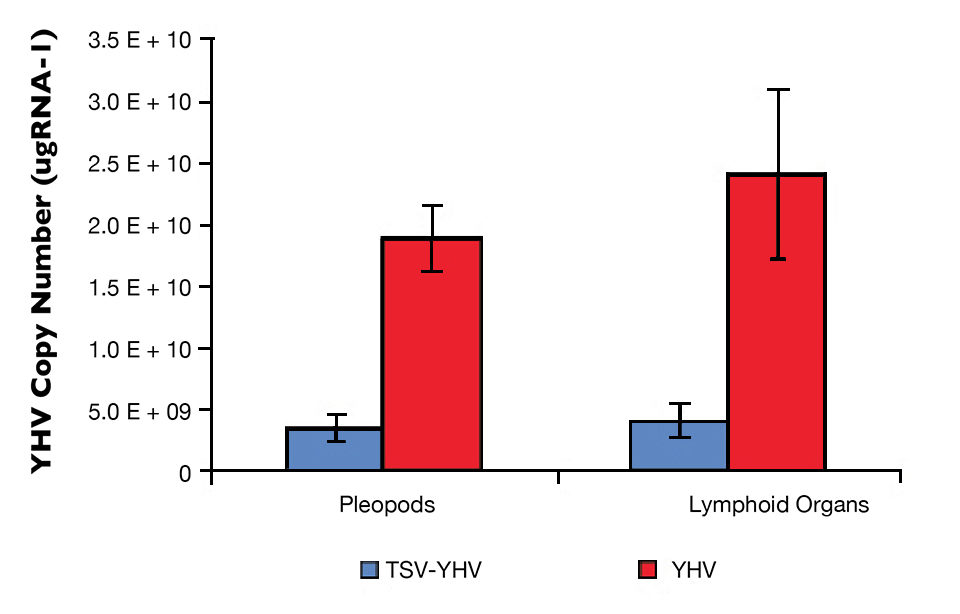
The YHV viral loads in pleopods and lymphoid organ samples showed similar results after 27, 37 and 47 days (Fig. 2) in the TSV-YHV and YHV groups. A five times higher YHV viral load was found in pleopods in the YHV group than in the TSV-YHV group (Fig. 4). The YHV copy number in the lymphoid organs of the TSV-YHV was 5.8 times lower than in the YHV group.
The TSV viral load in pleopod samples showed similar results 27, 37 and 47 days after exposure. This was typical of the TSV chronic phase, where most of the TSV viral particles are harbored in lymphoid organs and circulated in the hemolymph.
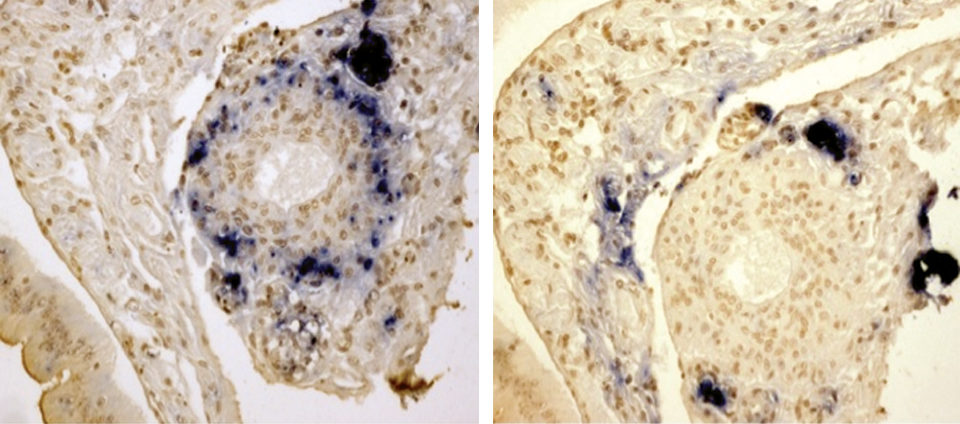
The results from the in situ hybridization confirmed the shrimp were infected with the two viruses. However, as expected, the distribution of the viruses was different. TSV was restricted to the L.O., whereas YHV was found in the cuticular epithelium, connective tissue and L.O. Interestingly, the TSV was distributed mainly in the L.O. tubules, while YHV seemed to be present in areas where TSV was not present and adjacent areas of the tubules including L.O. spheroids. The typical severe necrosis caused by acute YHV was not observed in the lymphoid organs of shrimp preinfected with TSV.
Viral interference
In this study, a clear effect of viral interference was observed when shrimp were initially exposed to TSV and then infected with YHV. This interaction of TSV on YHV infection reduced the mortality and, hence, an increase in survival was observed.
The higher final survival in the TSV-YHV group is explained in part by the lower YHV viral genetic load in pleopods and lymphoid organs. In addition, the restriction of YHV distribution in areas of L.O. where TSV was not present may have prevented this organ from experiencing the severe necrosis observed in the YHV group.
By histological analysis, similar results were observed. In the TSV-YHV group, YHV was present, however, the typical severe necrosis of the L.O. was not observed. It was observed in the positive control. The in situ hybridization results showed that the infection by the two viruses occurred in the L.O.
The same tissue can harbor the two viruses, but the regions of the L.O. infected first by TSV were not (or lightly) infected with YHV. The interaction between TSV and YHV was probably due to TSV interfering with the YHV infection.
Perspectives
YHV has been suspected in some shrimp-farming locations in the Western Hemisphere, and sporadic positive results by polymerase chain reaction testing suggested the presence of this etiological agent. In 2006, infectious YHV was reported on the Pacific coast of Mexico in wild and farm-raised shrimp. Thus, one of the possible explanations for the absence of yellow head disease in L. vannamei culture in the Americas could be the widespread presence of TSV before YHV arrived with white spot syndrome virus.
Pre-exposure of shrimp to TSV might block the entry of YHV by competing for the cell receptors. Even though YHV can infect tissues that TSV cannot, it is likely the critical lymphoid organ and cuticular epithelium tissues are essential for YHV pathogenesis. Thus, by blocking entry into those tissues, virus infection of other target tissues could be prevented. Additional work is needed to elucidate the whole blocking process at the molecular level.
(Editor’s Note: This article was originally published in the March/April 2013 print edition of the Global Aquaculture Advocate.)
Authors
-
Luis Fernando Aranguren
Colombian Corporation Center for Aquaculture Research
CENIACUA
Carrera 9B-113-60
Bogotá, Colombia -
Kathy Tang, Ph.D.
Department of Veterinary Science and Microbiology
University of Arizona
Tucson, Arizona, USA -

Donald V. Lightner, Ph.D.
Department of Veterinary Science and Microbiology
University of Arizona
Tucson, Arizona, USA
Tagged With
Related Posts

Health & Welfare
A comprehensive look at the Proficiency Test for farmed shrimp
The University of Arizona Aquaculture Pathology Laboratory has carried out the Proficiency Test (PT) since 2005, with 300-plus diagnostic laboratories participating while improving their capabilities in the diagnosis of several shrimp pathogens.

Responsibility
A look at various intensive shrimp farming systems in Asia
The impact of diseases led some Asian shrimp farming countries to develop biofloc and recirculation aquaculture system (RAS) production technologies. Treating incoming water for culture operations and wastewater treatment are biosecurity measures for disease prevention and control.

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 1
Indian shrimp hatcheries have experienced larval mortality in the zoea-2 stage, with molt deterioration and resulting in heavy mortality. Authors investigated the problem holistically.

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 2
Indian shrimp hatcheries have experienced larval mortality in the zoea-2 stage, with molt deterioration and resulting in heavy mortality. Authors considered biotic and abiotic factors. Part 2 describes results of their study.


