Feed conversion, survival improved over previous trials

Recirculating aquaculture systems (RAS) offer several advantages over traditional shrimp farming in coastal ponds. Water requirements are reduced significantly, thereby reducing the potential for pathogen introduction into the culture environment, and nutrient and biological pollution of the natural environment. In addition, an RAS can produce shrimp at an inland location away from sensitive coastal areas and closer to markets. However, higher capital and operating costs have been a barrier to the economic viability of RAS grow-out technology.
Recent research at the Oceanic Institute in Hawaii, USA, has focused on reducing production costs by simplifying recirculating system design, improving management strategies, and enhancing shrimp performance through selective breeding.
Grow-out trial
In a super-intensive grow-out trial, shrimp representing 27 families from the Oceanic Institute selective-breeding program were tagged and stocked in a raceway-based recirculating system. The RAS consisted of a 75-square-meter concrete raceway with system components that included a foam fractionator, oxygen generator, and air stones. Clear ultraviolet-protected plastic sheeting was used to cover the raceway as a biosecurity feature to reduce pathogen introduction by airborne vectors. The cover also served as an effective thermal insulator to maintain desirable wat-er temperatures.
Water use was minimized so that suspended particles, attached bacteria, and microalgae could be retained in the production system. This was important because shrimp reared in water with high concentrations of microalgae and microbial-detrital aggregates grow better than shrimp reared in filtered sea water. Also, suspended particles could serve as attachment sites for nitrifying bacteria.
A total of 22,600 juvenile shrimp with mean weight of 0.81 grams were stocked in the RAS at an initial density of 300 shrimp per square meter. The shrimp were fed a commercial 35 percent protein feed in the morning by hand and by belt feeders for the remainder of the day and evening.
Shrimp performance
After 14 weeks, 20,238 shrimp with a mean weight of 21 grams were harvested from the raceway. Final survival was 89.5 percent, and mean growth rate was 1.44 grams per week. The feed-conversion ratio was 1.49, with a final production of 5.7 kilograms per square meter (7.5 kilograms per cubic meter).
Batch weights by family at stocking and individual harvest weights were measureed to evaluate family performance. Seven shrimp families exhibited growth performance of 1.5 grams per week or more and at least 85 percent survival during the 14-week trial. One family had a mean weight gain of 24.7 grams (1.8 grams per week growth) and survival of 99.5 percent. Furthermore, 243 individuals (2.7 percent of the tagged shrimp harvested) exhibited growth rates of 2 grams per week. This group included representatives from 18 of the 27 families. Clearly, selective breeding will continue to play a significant role in ensuring good shrimp performance in these production systems.
Water quality
One hundred sixty l of water were used to produce 1 kg of shrimp. Water lost to evaporation and the removal of protein fractionator sludge was replaced at a rate of 0.34 percent per day.
The high mean water temperature of 28.8 degrees-C ± 0.1 S.E. throughout the 14-week trial supported rapid shrimp growth and could be attributed, in part, to the plastic cover that acted as a thermal insulator. The mean dissolved oxygen (D.O.) concentration was 5.48 milligrams per liter ± 0.07 S.E. With few exceptions, D.O. concentrations were maintained above 5.00 milligrams per liter throughout the trial.
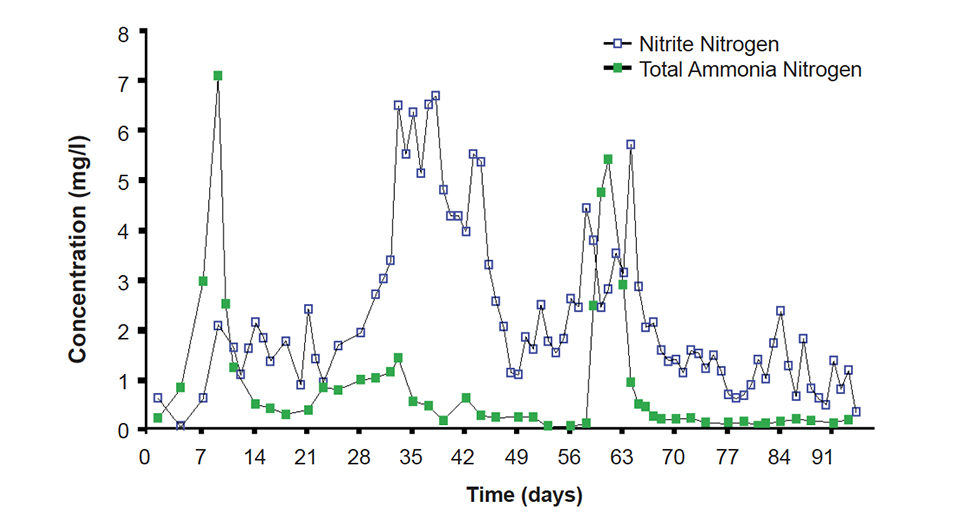
Mean total ammonia nitrogen concentration was 0.99 milligrams per liter ± 0.21 S.E. (Fig. 1), while mean nitrite concentration was 2.32 milligrams per liter ± 0.18 S.E. Ammonia and nitrite removal was accomplished by in situ microbes in the water column and attached to surfaces. No external biofilters were used during the trial.
Nitrifying bacteria
The initial fill water for the RAS was clear well water devoid of particles upon which nitrifying bacteria could attach. This likely accounted for the variable concentrations in total ammonia nitrogen and nitrite nitrogen observed throughout most of the trial. However, low and stable concentrations of these inorganic nitrogen species were observed during the last three weeks of the trial, when shrimp biomass was high.
On day 58, the mean total bacterial count using a highly sensitive “DAPT” DNA stain was 3.9 x 108 cells per milligrams. Fluorescence in situ hybridization techniques indicated that nitrifying bacteria of the genus Nitrospira were present at a concentration of 1.2 x 108 cells per milligrams and represented 31 percent of the total count.
Water analysis conducted on day 91 showed a total suspended solids concentration of 1,365 milligrams per liter . The particles provided significant surface area upon which nitrifying bacteria could attach. In addition, photoautotrophs were present in the water column, and mean net oxygen production was 3.1 mg oxygen per liter per hour. Data on RAS microbes suggests there is functional redundancy in terms of ammonia and nitrite removal, where photoautotrophs, nitrifying bacteria, and heterotrophic bacteria all play important roles.
Evolving performance
Results from this trial reflected advances over previous Oceanic Institute research. Water exchange, for example, was significantly less than previous trials with 1 to 2 percent daily exchange. Alternative management strategies allowed the elimination of costly external biofilters and other system components – aspirator-type aerators, degassing columns, and artificial substrates – and reduced production costs.
Feed conversion and survival also improved over previous trials, in which feed-conversion ratios were typically greater than 2 and survival ranged 70 to 85 percent. Although super-intensive trials at the Oceanic Institute have been conducted using stocking densities as high as 700 shrimp per square meter, the moderate stocking density used in this trial may provide a more consistent, reproducible result.
(Editor’s Note: This article was originally published in the July/August 2006 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Clete A. Otoshi
Oceanic Institute
41-202 Kalanianaole Highway
Waimanalo, Hawaii 96795 USA -
Carrie M. Holl, Ph.D.
Oceanic Institute
41-202 Kalanianaole Highway
Waimanalo, Hawaii 96795 USA -
Dustin R. Moss
Oceanic Institute
41-202 Kalanianaole Highway
Waimanalo, Hawaii 96795 USA -
Steve M. Arce
Oceanic Institute
41-202 Kalanianaole Highway
Waimanalo, Hawaii 96795 USA -
Shaun M. Moss, Ph.D.
Oceanic Institute
41-202 Kalanianaole Highway
Waimanalo, Hawaii 96795 USA
Tagged With
Related Posts

Health & Welfare
Add value to shrimp harvests: Darken environment to enhance shrimp color
Shrimp color is caused by pigments that play an important role in camouflage for survival. For farmed shrimp, proper coloration is generally achieved via synthetic astaxanthin in feed.

Health & Welfare
A comprehensive look at the Proficiency Test for farmed shrimp
The University of Arizona Aquaculture Pathology Laboratory has carried out the Proficiency Test (PT) since 2005, with 300-plus diagnostic laboratories participating while improving their capabilities in the diagnosis of several shrimp pathogens.

Aquafeeds
A look at India’s fish feed industry
India's fish-farming industry makes limited use of modern feeds, providing potential for the feed sector to grow. Commercial feeds are predominantly used for pangasius farming, followed by a rising popularity in carp culture.

Health & Welfare
A look at aquaculture in Guyana
With its large quantities of water and little industry to pollute it, Guyana has the potential to become a greater player in global aquaculture.


