Barcelona study evaluates the effect of feed supplements on immune indicators

Gilthead seabream (Sparus aurata) is the top aquacultured marine fish species in Europe, with over 70,000 metric tons (MT) produced in 2001. In colder regions of the Mediterranean, the species is affected by winter disease, a complex syndrome that has been related to cold stress-induced immune suppression.
A recent study at the Universidad Autónoma de Barcelona, Spain evaluated the effect of immune-enhancing feed supplements on the immune indicators of gilthead seabream during prolonged administration and a temperature and photoperiod ramp that simulated winter and early spring.
Nonspecific immune system
In fish, nonspecific immune mechanisms are a first line of defense that – in contrast to mammals – represents a significant part of the fish immune response. If pathogens pass the first barrier of the nonspecific immune system, which includes the skin and mucus, cellular and humoral components are triggered to kill the infectious agents.
When the nonspecific immune response fails to stop an infection, fish develop the disease. This leads to the induction of specific defense mechanisms mediated by macrophages and lymphocytes, and the production of antibodies. The nonspecific immune system plays a role in the activation of the specific immune response, which functions as an immunological memory to prevent new infections caused by past pathogens.
Nutrition and disease
Nutrition plays an important role in the development of an adequate immune response. The amount of nutrients supplied in the diet for normal growth may not be sufficient to support an optimal response by the immune system. Furthermore, antinutritional factors, mycotoxins, and nutritional imbalances can have adverse effects on the capability of fish to resist infection. Many studies have shown that health and immunology are dependent on an adequate supply of key nutrients like vitamins, phospholipids, essential fatty acids, nucleotides, trace minerals and carotenoids.
Immunostimulants
Several compounds, mostly derived from the cell envelopes of microorganisms, have been identified as immunostimulants. The efficacy of immunostimulants to improve resistance to stress and disease strongly depends on the type of compound and supply of adjuvant nutrients essential to support the build-up of the immune system.
Experimental design
Seabream of 70 to 80 grams were stocked in 500-liter tanks (two tanks per treatment) at a density of 6 kilograms per cubic meter. Fish were fed twice daily according to standard feeding tables. Feeding was stopped when the fish refused to eat. Nitrite, ammonia, and pH were kept within acceptable limits for seabream. During the trial, no diseases or infections were observed.
Blood sampling, preparation
Blood was sampled from eight fish per treatment at the start of the test (S1), after four weeks feeding at a constant temperature of 18 degrees-C (S2), after gradual temperature decrease to 11 degrees C (S3), after two weeks at 11 degrees C (S4), and after gradual temperature increase up to 18 degrees-C (S5). The photoperiod simulated the natural photoperiod, starting as 13 hours light per 11 hours dark and decreasing progressively to 11 light per 13 dark at the end of the experiment.
For blood sampling, fish were placed in anesthetic and sampled through caudal puncture with a syringe. Fresh blood was placed in heparinized tubes for nitro-blue tetrazolium determination and lymphocyte count. The rest of the blood was left to clot at 4 degrees-C for two hours. After removing the clot by centrifugation, the serum was aliquoted and deep frozen until the analytical determinations of lysozyme, complement, and agglutination (Fig. 1).
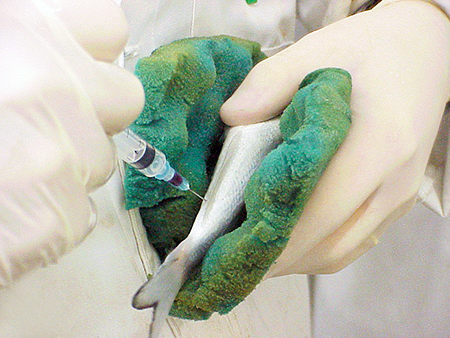
- Lymphocyte number: Count of white blood cells responsible for proliferation and synthesis of antibodies following an infection.
- Phagocytosis: Process responsible for the elimination of infectious organisms, initiated by a liberation of free radicals by granulocytes during the so-called “respiratory burst,” which results in a strong microbicidal action. Evaluated by the color change of nitro blue tetrazolium.
- Lysozyme: Enzyme capable of killing bacteria by breaking the peptidoglycan layers of the bacterial cell envelope.
- Complement: Complex of soluble factors involved in the direct elimination of infectious organisms and enhancement of the specific immune system.
- Agglutination: Specific mechanism induced by agglutinating molecules in the serum (immunoglobulins and lectins), which may agglutinate antigens and thus prevent the activity of pathogens.
- Immunoglobulins: Proteins in IgM fish that act specifically against pathogens.
Experimental feeds
A standard diet for sea bream (48 percent protein, 17 percent fat) incorporating herring meal, soybean flour and meal, wheat flour, wheat gluten, fish oil, and a 1 percent vitamin-mineral premix was used as a base formulation. In a control diet, 2.5 percent cellulose was added as filler, while the experimental feeds used three feed supplements at 2.5 percent inclusion:
- Glucan, a supplement based on purified betaglucan
- Supplement based on treated yeast
- Aquastim-F, a commercial feed supplement with a blend of natural immunostimulating compounds (including betaglucans and mannans) and nutrients (vitamins, amino acids, nucleotides, micronutrients, and antioxidants).
Immunostimulatory activity
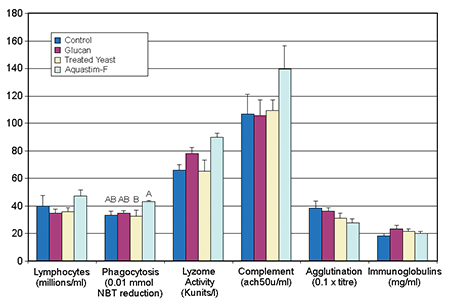
After one month of feeding, fish were sampled at the control temperature. Significant differences were not easily detected due to individual variability. Nevertheless, the diet with the commercial feed supplement gave a higher degree of stimulation in the nonspecific defense indicators of lymphocyte count, phagocytic activity, lysozyme activity, and complement than the other feeds.
Lysozyme activity was stimulated in the groups fed the Aquastim- and glucan-based feeds. No changes were observed in specific responses (agglutination activity and immunoglobulin levels) due to the addition of the feed supplements, which could be expected from the absence of infections during the trial. The diet containing the commercial feed supplement showed better immunostimulatory activity at the control temperature (Fig. 2).
Effect of cold shock
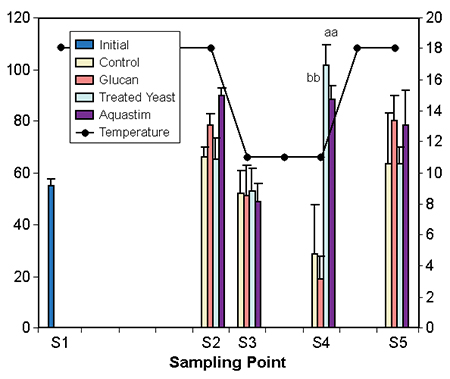
The decrease of temperature from 18 to 11 degrees-C in seven days showed a clear effect in the levels of both nonspecific and specific immune indicators. The temperature change decreased complement and lysozyme (Fig. 3), increased lymphocytes and phagocytosis activity (Fig. 4), and decreased agglutination activity and total immunoglobulins.
Tort et al. (1998) and Padros et al. (1996) observed a similar decrease of complement, plasma lysozyme, and agglutination activity in seabream during an outbreak of winter syndrome in cage cultures in northeast Spain. Increased immunocompetence, which suggests a better degree of protection, was noted in animals fed the diets containing the glucan and Aquastim.
Maintaining phagocytosis and lysozyme activity
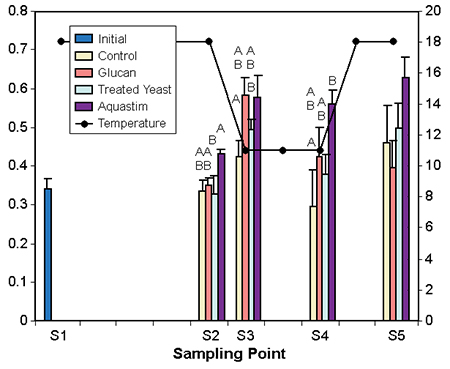
Fish maintained at 11 degrees-C for two weeks after the cold shock showed lower values in phagocytosis and lysozyme activity. The experimental feed supplements enhanced these immune indicators differently. The Aquastim-based feed produced enhanced levels of both phagocytosis and lysozyme activity. The glucan- and treated yeast diets resulted in a moderate increase of phagocytosis, and only the treated yeast boosted lysozyme activity.
No differences among groups were observed for agglutination and immunoglobulin levels. These parameters recovered during the course of the cold period regardless of the dietary treatment. This indicated that during prolonged exposure to cold, feed supplements can maintain levels of phagocytosis and lysozyme activity.
Figs. 3 and 4: Effect of temperature/photoperiod ramps on immune indicators of gilthead seabream fed nutritional supplements. Temperature (degrees-C) follows the right axis. Data represent average of eight fish. Different letters within the sampling denote significant differences at p < 0.5 (abc) or p > 0.1 (ABC) Tuckey’s HSD test.
Restoring immunocompetency during temperature recovery
After recuperation from 11 to 18 degrees-C in one week, followed by one more week at 18 degrees-C, specific defense levels recovered and nonspecific indicators showed a recovery that was not complete for complement. The high variability between individuals during this phase of the trial did not allow detecting dietary effects on the immune parameters. However, higher phagocytosis activity seemed to persist in the group whose feed contained the commercial feed supplement, and agglutination levels increased at the end of the temperature rise regardless of the dietary treatment.
Conclusion
The simulation of winter temperature/photoperiod in the laboratory affects both the nonspecific and specific immune indicators of gilthead sea bream. During this study, the inclusion of a feed supplement based on a blend of immune-enhancing compounds and nutrients boosted the nonspecific immune parameters of gilthead sea bream prior to and during a temperature/photoperiod ramp. This boost was less pronounced when feed supplements were based on pure immune stimulants such as betaglucans or treated yeast.
(Editor’s Note: This article was originally published in the October 2002 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
-
L. Tort
Universidad Autónoma de Barcelona
Department of Cell Biology, Physiology, and Immunology
Barcelona, Spain -
J. Rotllant
Universidad Autónoma de Barcelona
Department of Cell Biology, Physiology, and Immunology
Barcelona, Spain
Tagged With
Related Posts

Health & Welfare
Advances in fish hatchery management
Advances in fish hatchery management – particularly in the areas of brood management and induced spawning – have helped establish aquaculture for multiple species.

Health & Welfare
Algae alternatives serve in larval rearing of sea bream
Sea bream fry are produced using the greenwater technique in which microalgae are added to larval-rearing tanks during the first 20 to 30 days after hatching.

Health & Welfare
By-the-numbers nutritional bioenergetics for optimal feeding
Using a series of equations, energy and protein requirements for every fish species can be calculated and adapted to changing conditions.

Health & Welfare
Computer modeling helps grow fish
For farmers, computer modeling helps analyze crop production and environmental effects. For managers, it assists with carrying capacity assessments and licensing issues.


