Alkalinity should not greatly exceed hardness to avoid high pH in response to photosynthesis

Total alkalinity and total hardness are basic water quality variables in aquaculture. For simplicity, they can be referred to as simply alkalinity and hardness. Alkalinity is an index of the capacity of water to neutralize acidity that is usually expressed in milligrams per liter of equivalent calcium carbonate. Hardness, the sum of the calcium and magnesium concentrations, also is usually expressed the same way.
The calculation of alkalinity and hardness in terms of equivalent calcium carbonate is illustrated by the examples in Fig. 1. In the second example, hardness is estimated from calcium and magnesium concentrations, although it is more common to measure hardness by titration with ethyelenediaminetetraacetic acid.
Neutralizing capacity
The capacity of natural water to neutralize acidity or its alkalinity resides primarily in the amounts of bicarbonate and carbonate dissolved in it. Water with pH below 4.5 does not contain bicarbonate and has a source of acidity stronger than carbon dioxide. This is the reason the color change of methyl orange indicator from yellow to orange at pH 4.5 can be used to signal the end point of alkalinity titration.
Such a low pH in water usually is from sulfuric acid that originates from the oxidation of naturally occurring iron sulfite in geological material or from acidic rain. Water with pH of 4.5-8.3 contains both bicarbonate (alkalinity) and carbon dioxide (acidity). Water with a pH above 8.3 contains carbonate and bicarbonate, but no measurable carbon dioxide. The relationships of carbon dioxide, bicarbonate, and carbonate to pH are illustrated in Fig. 2.
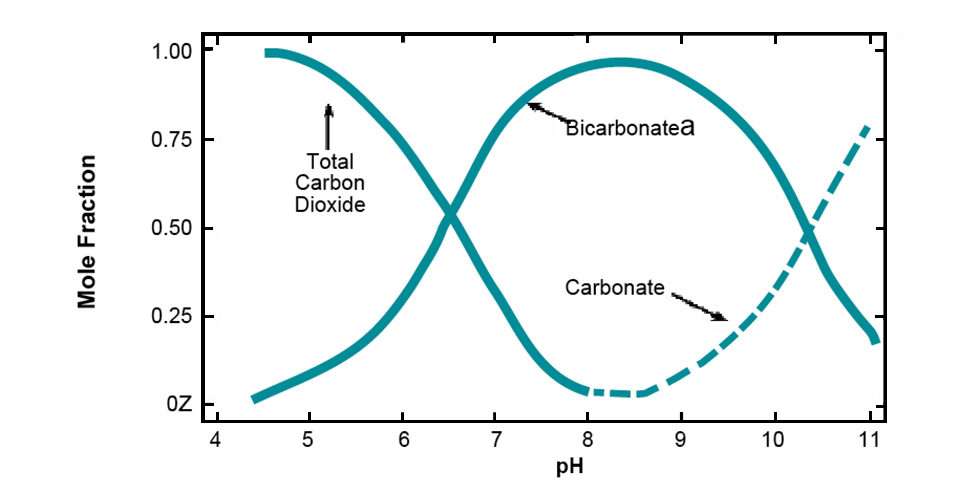
The main source of bicarbonate in natural water is the dissolution of the common minerals limestone, calcium silicate, and feldspars in soil, sediment, and other geological materials. Dissolved carbon dioxide facilitates the dissolution. When limestone dissolves, both alkalinity and hardness increase in chemically equivalent quantities. The dissolution of calcium silicate also provides both alkalinity and hardness, but no hardness results when feldspars dissolve.
Effect of photosynthesis
Photosynthesis by aquatic plants removes carbon dioxide from water and causes pH to rise. Free carbon dioxide is depleted at pH 8.3, and plants begin to remove carbon for photosynthesis from bicarbonate. This process causes carbonate to increase in the water, and because carbonate is a strong base, the pH continues to increase.
In water with appreciable calcium, precipitation of carbonate as calcium carbonate tends to limit the pH rise. In normal waters, where hardness is nearly equal to or greater than alkalinity, pH seldom rises above 9.5. However, if photosynthesis is especially intense or waters contain appreciably more alkalinity than hardness, pH can rise to 12.
Concentration variations
The concentrations of alkalinity and hardness vary greatly in different freshwater sources. Surface waters in humid regions with thin or highly leached soils usually have less than 15-20 mg/l of alkalinity and hardness. Surface waters in humid regions where soils are more fertile but do not contain carbonate usually have alkalinity and hardness concentrations of 20-50 mg/l. In humid regions where soils contain carbonate, alkalinity and hardness often exceed 100 mg/l in surface water.
Evaporation exceeds precipitation in arid regions, and concentrations of major ions increase in surface waters. Alkalinity often reaches 200-300 mg/l, and hardness can be much higher.
Seawater has less-variable concentrations of alkalinity and hardness than freshwater. In seawater, normal values of alkalinity are about 140 mg/l, while hardness is about 6,000 mg/l.
Ground water from wells and springs also can vary greatly in alkalinity and hardness. Water from aquifers in geological material containing limestone can have concentrations of 500 mg/l or more for both variables. When such waters are placed in ponds or other open vessels, calcium carbonate often precipitates as alkalinity and hardness concentrations equilibrate with existing conditions. Some ground water has high concentrations of alkalinity, but low concentrations of hardness and especially calcium. Such waters develop a very high pH in response to photosynthesis.
Management in aquaculture systems
In aquaculture ponds, it is usually desirable to have alkalinity and hardness concentrations above 50 mg/l for fish and 80 mg/l for crustaceans. Waters should not have appreciably more alkalinity than hardness to avoid high pH in response to photosynthesis. Higher hardness is not especially troublesome. However, water with alkalinity and hardness above 200 mg/l may not respond well to fertilization. The high pH and calcium concentration in such waters favor rapid precipitation of calcium phosphate.
In hatcheries, precipitation of calcium carbonate from ground water sources high in alkalinity and hardness can be harmful to eggs and larvae of aquaculture species. Low calcium concentrations in water for hatcheries often lead to poor egg hatchability.
Pond water with low alkalinity and hardness can be treated with agricultural limestone or lime to raise the concentrations of these variables and increase pH. The treatment rate in ponds usually is based on pond bottom soil pH (Table 1). In hatcheries, liming materials usually are dispersed into the incoming water continuously by mechanical dispensers.
Boyd, Adjusting agricultural limestone, Table 1
| Soil pH | Limestone Application (kg/ha) |
|---|---|
| 7.1 or above | 0 |
| 7.0-6.6 | 500 |
| 6.5-6.1 | 1,000 |
| 6.0-5.5 | 2,000 |
| 5.4 or less | 3,000 |
Waters with excessive alkalinity and hardness for use in hatcheries can be aerated with mechanical aerators or allowed to fall through a gas-stripping tower to remove carbon dioxide, and held in a basin until the calcium carbonate precipitate falls out.
Excessively hard water can be treated by ion exchange to remove calcium and magnesium. This technique usually is too expensive for treating waters used in aquaculture, however.
The hardness in water with low calcium and high alkalinity should be increased to approximately the same concentration as the alkalinity by application of calcium sulfate, also known as gypsum. Roughly 2 mg/l of calcium sulfate are needed to increase the hardness by 1 mg/l. Calcium chloride also can be used to increase calcium concentration, but it usually is more expensive than gypsum.
(Editor’s Note: This article was originally published in the March/April 2007 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Author
-

Claude E. Boyd, Ph.D.
Department of Fisheries and Allied Aquacultures
Auburn University
Alabama 36849 USA[97,117,100,101,46,110,114,117,98,117,97,64,49,101,99,100,121,111,98]
Tagged With
Related Posts

Responsibility
Accuracy of custom water analyses varies
The reliability of trace element analyses reported by custom laboratories cannot be checked by simple techniques, and results may not always be accurate. One should check the reliability of major ion analyses by determining the charge balance and comparing the measured total ion concentration with the total ion concentration estimated from conductivity.

Responsibility
Pond fertilization reevaluated
Although higher rates have been promoted, pond fertilization ratios of 2:1 or 1:1 nitrogen to phosphorus should be maintained in older ponds for food fish production.

Responsibility
Appraising pond liners for shrimp culture
The use of plastic-lined ponds by shrimp farmers can significantly improve production efficiency, support more production cycles per year, and higher mechanical aeration rates and stocking densities. The capital cost of lining ponds can be very significant, so a thorough feasibility analysis is recommended when considering this production tool.

Responsibility
Carbon dioxide: Waste, nutrient
Carbon dioxide is both a nutrient and a waste product in aquaculture. Some pond managers feel that application of organic matter to provide additional carbon dioxide can reduce pH and control blue-green algae.

