Excessive copper concentrations problematic for phytoplankton, aquatic plants

Metals are more numerous than non-metals, making up 84 of the 103 known elements. The major cations in water – sodium, potassium, calcium and magnesium – are metals, and the common trace metals in water are aluminum, beryllium, cadmium, chromium, cobalt, copper, iron, lead, manganese, mercury, nickel, selenium, silver, tin and zinc.
Several of these – cobalt, copper, iron, manganese, zinc and possibly selenium – are required by plants, animals or both. However, all of the aforementioned trace metals can be toxic at elevated concentrations.
Characteristics
The ultimate sources of trace elements are minerals in the earth’s crust. Aluminum, iron and manganese make up 8.3, 6.2 and 0.1 percent, respectively, of the weight of the crust. The other trace metals are present in much smaller quantities but can be abundant in certain deposits.
Trace elements dissolve in water, but their concentrations are typically quite low (Table 1). The major factor favoring the solubility of trace metals from their parent minerals is low pH. Low redox potential also greatly increases the solubility of iron, manganese and a few other trace metals.
Boyd, Typical concentrations and maximum safe concentrations, Table 1
| Trace Metal | Concentration (μg/L) Typical for Freshwater | Concentration (μg/L) Average for Seawater | Maximum Safe Concentration (μgIL) Soft Water* | Maximum Safe Concentration (μgIL) Hard Water (including Marine Water) |
|---|
Trace Metal | Concentration (μg/L) Typical for Freshwater | Concentration (μg/L) Average for Seawater | Maximum Safe Concentration (μgIL) Soft Water* | Maximum Safe Concentration (μgIL) Hard Water (including Marine Water) |
|---|---|---|---|---|
| Aluminum | Trace-100.00 | 10.00 | 1,000.00 | 1,000.00 |
| Beryllium | 0.01-1.00 | 0.0006 | 11.00 | 1,100.00 |
| Cadmium | 1.00-5.00 | 0.11 | 25.00 | 70.00 |
| Chromium | 1.00-50.00 | 0.05 | 100.00 | 100.00 |
| Cobalt | 1.00-10.00 | 0.50 | 50.00 | 50.00 |
| Copper | 5.00-50.00 | 3.00 | 25.00 | 70.00 |
| Iron | Trace-500.00 | 10.00 | 1,000.00 | 1,100.00 |
| Lead | 1.00-20.00 | 0.03 | 200.00 | 4,000.00 |
| Manganese | Trace-250.00 | 2.00 | 1,000.00 | 1,000.00 |
| Mercury | Trace-0.02 | 0.03 | 0.10 | 0.10 |
| Nickel | 5.00-25.00 | 2.00 | 50.00 | 400.00 |
| Selenium | 0.10-5.00 | 4.00 | 20.00 | 20.00 |
| Silver | 0.01-0.30 | 0.30 | 0.05 | 0.10 |
| Tin | 1.00-20.00 | 3.00 | – | – |
| Zinc | 10.00-100.00 | 10.00 | 50.00 | 100.00 |
* For pH above 7.0.
Trace metals have many industrial uses. They are released into the atmosphere when coal is burned, and coal ash is high in certain trace metals. Thus, pollution is a major source of trace metals in water.
Trace metals in aquaculture
Unexplained aberrations in the survival and growth of aquaculture species often elicit concerns about excessive trace metal concentrations. Source water for aquaculture facilities can contain trace metals from natural sources and/or pollution. Feeds, fertilizers, algicides and some other substances used in pond management also contain trace metals. Trace metals can be exchanged between water and bottom soil. The soil can be either a sink or a source for individual trace metals.
The chemistry of dissolved trace metals is rather complicated. The concentration of the ionic form of a particular trace metal in water is determined by the solubility of its controlling mineral. Most minerals containing trace metals decrease greatly in solubility as pH increases. Metal ions in solution react with water, anions and organic matter to form hydrolysis products, ion pairs and coordination compounds, respectively, and greatly increase the total concentration of metals in solution.
Potential toxicity
Plants absorb the ionic forms of metals. This disrupts the equilibrium between the metal ion and its combined forms, and in response, the controlling mineral dissolves to release more of the ionic form. These processes are summarized using zinc as an example in Fig. 1.
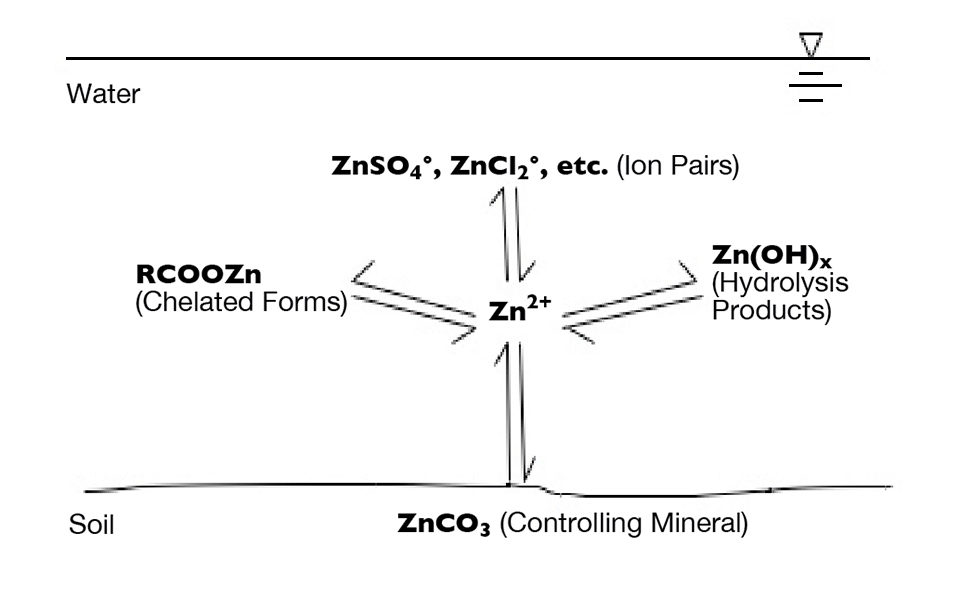
Excessive concentrations of trace metals, especially copper, are toxic to phytoplankton and other aquatic plants. Chelated copper compounds often are used as algicides, especially in alkaline water, where the high pH depresses the concentration of copper ion Cu2+, the form toxic to plants.
The ionic forms of trace metals also are toxic to fish and other aquatic animals, but soluble, chelated trace metals and other combined forms are relatively non-toxic. Chelated copper algicides can be used in acidic water to increase total copper concentration enough to kill algae without increasing the copper ion concentration to a level toxic to fish or shrimp.
In aquaculture, the greatest concern with respect to trace metals is their possible pollution of water supplies, resulting in mortality of the culture species or possible contamination of the culture species with residues of heavy metals.
Trace metal concentrations vary widely in pond bottom soils. However, there is no evidence that natural ambient concentrations of trace metals have caused problems in aquaculture ponds with slightly acidic to alkaline bottom soils and water. In ponds constructed in acid sulfate soils, concentrations of various trace metals, especially aluminum, have been associated with low survival and production. Therefore, sites with acid sulfate soils should generally be avoided for aquaculture.
However, heavy liming of ponds with acid sulfate soils and routine liming of less acidic ponds can maintain soil pH above 7 and assure that total alkalinity is 50 mg/L or more. These conditions prevent potentially toxic concentrations of aluminum and other trace metals. The natural basic reaction of seawater lessens the opportunity for trace metal toxicity from natural sources.
Pollution
The most common cause of toxic levels of trace metals is pollution. Aquaculturists should familiarize themselves with sources of trace metal pollution near their farms and avoid using contaminated water. It is very difficult to assess the concentrations of trace metals in a particular water. The ionic form of a trace metal is toxic, but analytical methods for trace metals measure the total concentration of trace metals.
Depending on pH, total alkalinity, salinity and dissolved organic matter concentration, the proportion of the ionic form to the total concentration can vary from less than 5 percent to more than 90 percent. Thus, the total concentration necessary to produce a toxic concentration varies greatly with water quality.
For example, in water with 5 mg/L total alkalinity and pH of 5.5 to 6.5, a total copper concentration above 0.01 mg/L would likely be toxic to most species of fish, but in water with 8 to 8.5 pH and 100 mg/L total alkalinity, a total copper concentration of 0.25 mg/L would not harm the same species. Aluminum toxicity is of concern in water with pH below 5.5, but in water with pH 7 or greater, aluminum toxicity is not an issue. As a rule, trace metal toxicity decreases with increasing pH, total alkalinity and salinity.
The maximum safe concentrations of trace metals for warmwater species are summarized in Table 1. These values are for total trace metal concentrations instead of metal ion concentrations. The data for freshwater assumes a pH of 7 or more. The safe concentrations of many of these trace metals would be considerably less at lower pH. Safe concentrations for coldwater species also would be lower than those shown.
Limited problems
It is doubtful that trace metals are a cause of stress or mortality of culture species in most ponds. The exceptions are ponds with low alkalinity, those with acidic water, ponds receiving water polluted with one or more trace metals, and those accidentally treated with excessive copper sulfate for algae control.
In hatcheries, chelating agents such as ethylenediaminetetracetic acid can be dissolved in the water at 5 to 10 mg/L as a precaution against trace metal toxicity.
There is relatively little information available on the trace element composition of aquaculture species. Trace metals such as iron, manganese, zinc and copper are needed by plants. Research has not demonstrated that the inclusion of these elements in fertilizers stimulates phytoplankton productivity. However, many authors have suggested that iron fertilization can be beneficial in ponds filled with brackish water or seawater.
(Editor’s Note: This article was originally published in the July/August 2009 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Author
-

Claude E. Boyd, Ph.D.
Department of Fisheries and Allied Aquacultures
Auburn University
Alabama 36849 USA
Tagged With
Related Posts

Responsibility
Addressing safety in Latin America’s tilapia supply chain
Over the last decade, the experience gained by many tilapia farmers combined with proficient programs implemented by local governments have significantly improved tilapia production in various Latin American countries like Colombia, Mexico, Ecuador and other important tilapia producers in the region.

Responsibility
Assessing groundwater quality in aquaculture
Those interested in using groundwater for aquaculture should perform a thorough chemical analysis of the water. Several problems related to groundwater use in hatcheries and holding or transport vessels can be alleviated by degassing or aeration.

Responsibility
Calcium and magnesium use in aquaculture
Aquatic plants and animals get the essential nutrients calcium and magnesium from water and food. Calcium concentrations impact the hydration and development of eggs in a hatchery, where calcium carbonate precipitation can be troublesome.

Intelligence
Land-based macroalgae farming
Land-based cultivation of macroalgae can minimize impacts on wild macroalgae stocks while reducing harvest costs and controlling quality. Integration of macroalgae with land-based fish culture systems could reduce capital and operating costs.

