Levels naturally rise in the afternoon hours
Natural waters not influenced by high levels of biological activity seldom have pH above 8.5, but in fish or shrimp culture, the pH of pond waters can increase to 9 or above. At such levels, increasing nonionized ammonia in the water can become toxic, and general chemical imbalance in the culture animal can result in mortaility.
Although high pH is commonly observed, many aquaculturists are unaware of both its causes and methods to combat it.
Carbon dioxide
Carbon dioxide is an important reactant in photosynthesis by green plants and a major byproduct of respiration by all organisms. Carbon dioxide causes an acidic reaction in water that produces hydrogen ions and bicarbonate.
In photosynthesis, the carbon in carbon dioxide is converted to organic carbon in the form of simple sugar, and molecular oxygen is released. In respiration, sugar (carbohydrate) is oxidized to carbon dioxide and water.
Phytoplankton
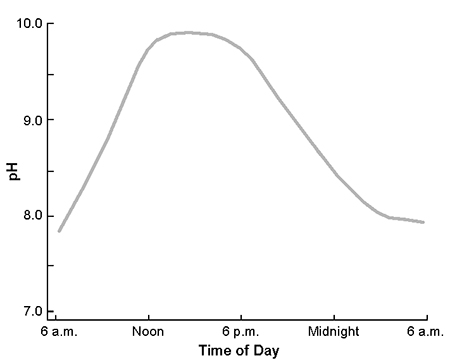
During daylight, phytoplankton in aquaculture ponds normally remove carbon dioxide through photosynthesis faster than respiration by the phytoplankton and other aquatic organisms releases carbon dioxide into the water. At night, photosynthesis stops because of the absence of light, but respiration continues and carbon dioxide concentration increases. As a result, the pH of pond water increases in daylight and decreases at night. In many cases, there can be a difference of two or more units between morning and afternoon pH (Fig. 1).
Bicarbonate and alkalinity
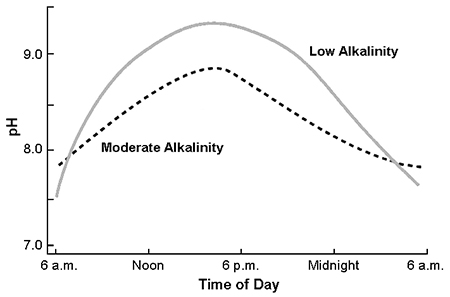
Bicarbonate, the main source of alkalinity in most natural waters, tends to buffer water against pH change. Water with low alkalinity naturally has a lower pH than water of high alkalinity. However, in aquaculture ponds, the afternoon pH increase normally is smaller in ponds with high alkalinity because of buffering by bicarbonate (Fig. 2). Nevertheless, even well-buffered water can have pH of 9 or more when dense phytoplankton blooms occur.
Conditions for high pH
Two situations are especially conducive to high pH. In new ponds or at the beginning of a crop in ponds subjected to several days of dryout, there is a low concentration of reactive organic matter in the sediment and water. Under these conditions, heterotrophic bacteria produce little carbon dioxide. Nutrients are added to ponds early in the crop cycle to stimulate phytoplankton, which quickly remove carbon dioxide from the water and cause pH to rise.
Because the phytoplankton community expands and heterotrophic activity does not return carbon dioxide to the water as fast as it is being used by plants, there is a net loss of carbon dioxide from the water. This increases pH over time. Afternoon pH can rise above 9.5, then remain over 9, even at night.
The second situation involves water with high alkalinity and a low concentration of calcium. When pH increases to 8.3, free carbon dioxide is expended, and plants begin to obtain carbon from bicarbonate. Part of the carbonate produced in this reaction hydrolyzes to increase hydroxide concentration, which causes pH to rise.
Calcium ions react with carbonate to form calcium carbonate, which is of limited solubility and precipitates. In water with a low calcium concentration, pH can increase above 10 when photosynthesis proceeds rapidly, because carbonate accumulates to a high concentration.
Treatments
Several treatments can successfully control high pH in aquaculture ponds.
Agricultural limestone
Ponds with total alkalinity below 50 milligram per liter or bottom soil pH below 7 should be treated with agricultural limestone. This will improve total alkalinity and increase the buffering capacity of the water.
Organic matter
Organic matter can be added to ponds to stimulate heterotrophic communities and release carbon dioxide to lower pH. Care must be used to prevent dissolved oxygen depletion.
Gypsum and calcium chloride
Calcium sulfate, often called gypsum, can be applied to increase calcium ion concentration, favor precipitation of carbonate, and decrease pH in ponds. Dose is determined as follows:
Calcium chloride can be used as a substitute for gypsum. Neither calcium sulfate nor calcium chloride is highly soluble in seawater.
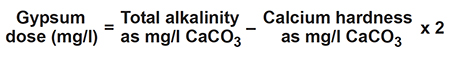
Alum and sulfuric acid
Some pond managers apply aluminum sulfate (alum), which forms sulfuric acid in water, or sulfuric acid to lower pH. Care should be taken to avoid adding too much acid and causing a dangerously low pH.
Dyes and water exchange
Dyes or colloidal clay have also been used in small ponds to increase turbidity, reduce light availability for photosynthesis, and lower pH. However, water exchange often is the only practical emergency measure for lowering pH.
pH measurement

The best way to measure pH is to use a standard pH meter and glass electrode (Fig. 3). Small pocket pH meters also provide relatively accurate pH estimates. Test kits for measuring pH usually read 0.5 to 1 unit too high.
In assessing pH, it usually is necessary to take samples both at dawn, when pH is lowest, and in mid-afternoon, when pH is highest. The pH is typically highest near the water surface and lowest near the pond bottom. Samples should be analyzed immediately for pH, for pH can change drastically during sample storage.
Conclusion
It is important for pond managers to understand that afternoon pH in the surface waters of their ponds often rises to 8.5 to 9.5. This is a normal event and usually causes no trouble. High pH is problematic only if it exceeds 9.5 in the afternoon, and remains at or near this level during the night.
Several treatments are used to control high pH in aquaculture ponds, including agricultural limestone, organic matter, gypsum, alum and dyes. Where high pH is limited to surface water, fish and shrimp can move into deeper water to escape exposure.
(Editor’s Note: This article was originally published in the June 2002 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Author
-

Claude E. Boyd, Ph.D.
Professor, Department of Fisheries and Allied Aquacultures
International Center for Aquaculture and Aquatic Environments
Auburn University
Auburn, AL 36849 USA
Tagged With
Related Posts

Responsibility
Light penetration in water
Light penetrating water is scattered and absorbed exponentially as it passes downward. The presence of dissolved organic matter and suspended solids further impedes light penetration, and different types of solids absorb different wavelengths.

Responsibility
Secchi disk visibility: Correct measurement, interpretation
It would be difficult to find a pond aquaculture worker who has not measured Secchi disk visibility or at least seen someone measure it.

Responsibility
Chemical fertilizers in pond aquaculture
Chemical fertilizers are frequently used in pond aquaculture to stimulate phytoplankton productivity and enhance the availability of natural food organisms.

Responsibility
Liming materials for aquaculture
Liming materials neutralize acidity and increase pH in pond bottom soil and water. They also react with carbon dioxide to form bicarbonate and release calcium and magnesium, increasing both alkalinity and hardness concentrations in water.

