Nitrification an integral microbial process in RAS

A significant development in the optimization of high-intensity shrimp production systems in the United States is the movement toward closed or recirculating aquaculture systems (RAS).
The Oceanic Institute in Waimanalo, Hawaii, USA, currently cultures Pacific white shrimp (Litopenaeus vannamei) under intensive conditions in recirculating systems that rely solely on the microbial community for the removal and sequestration of dissolved nitrogen species that are toxic to shrimp.
Shrimp RAS
The use of recirculation for shrimp production represents a major paradigm shift from methods that commonly rely on open coastal ponds and flow-through water exchange as a management strategy to improve water quality. Recirculation alleviates problems associated with effluent discharge and reduces the risk of pathogen introduction from water-borne vectors.
Recirculating systems also have the added advantage of moving shrimp production inland from coastal areas and within closer proximity to markets. Inland recirculating systems ultimately depend on a highly functional in situ microbial community to maintain acceptable water quality. In addition, bacteria, microalgae, and microbial-detrital aggregates provide supplemental nutrition for the shrimp.
RAS research
Oceanic Institute research on recirculation indicates that the microbial community changes throughout grow-out from stocking to harvest, both in terms of biomass and community function. Typically, the bacteria exhibit a characteristic increase in cell density from 10 million cells per milliliter at the start to roughly 300 million cells per milliliter within the first five weeks. Correspondingly, the biological oxygen demand in the water column increases with time (Figs. 1 and 2). Results from a light/dark trial suggested that microalgae in raceways with light are important for the removal of nitrate and ammonia.
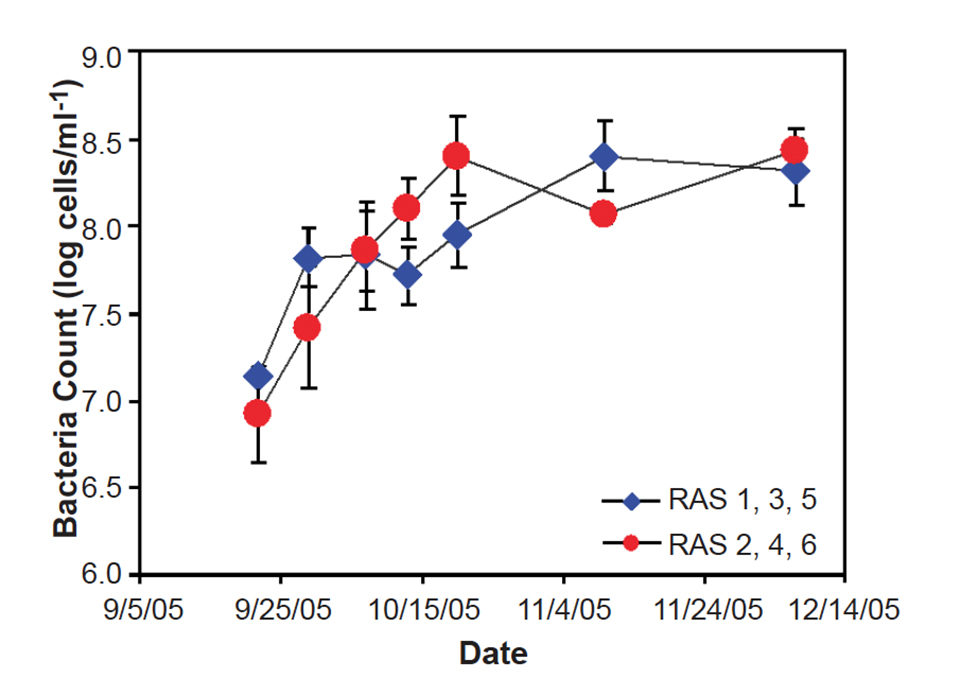
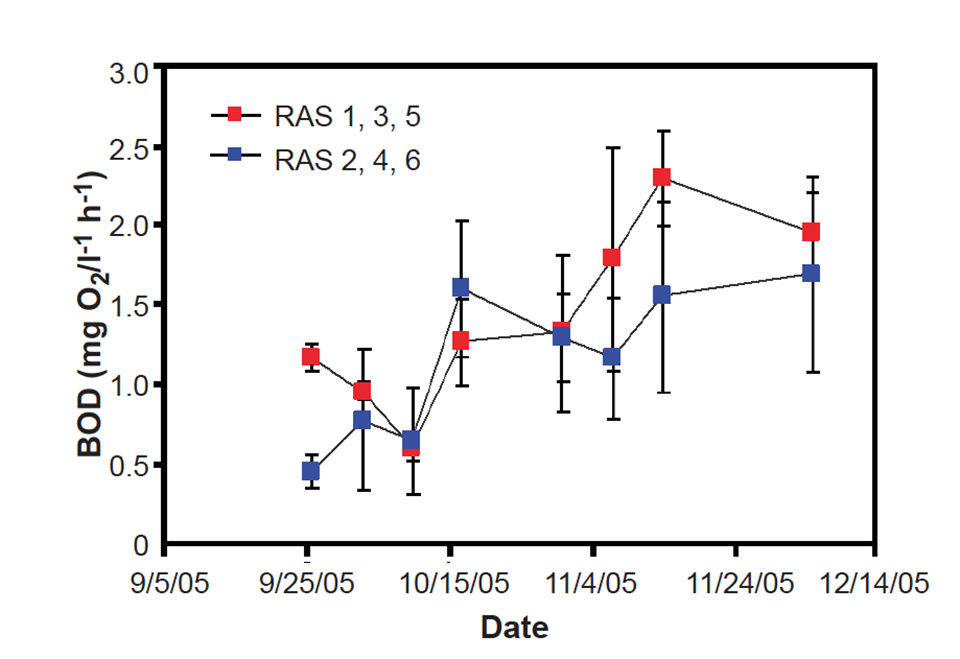
In addition, microalgae incorporate carbon into the particulate fraction and therefore retain nitrogenous nutrients in algal biomass. Interestingly, measurements of the natural abundance of nitrogen and carbon-stable isotope show that shrimp did not incorporate these small (less than 3 μ) algal cells as a supplementary food source during this study. However, shrimp survival was higher in the presence of light, suggesting the importance of phytoplankton to system stability.
Trophic states
Recirculation systems are often categorized as net phototrophic, autotrophic, or heterotrophic. However, the authors’ stable isotope data suggests this distinction is not clear and that all three trophic states exist simultaneously when light is available. Fig. 3 shows the carbon-stable isotope signature of three discrete size fractions of water column particles from the Oceanic Institute system.
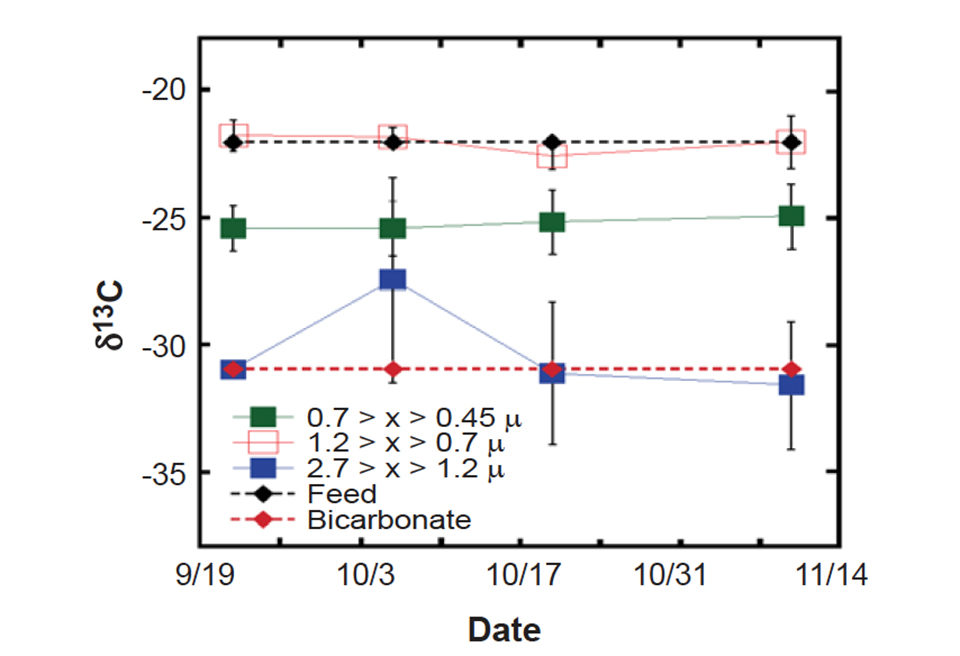
The isotopic signature of the largest fraction clearly reflects photosynthetic incorporation of bicarbonate. Heterotrophic particles, as evidenced by a carbon signature closely resembling that of the feed, is seen in the 1.2 > x > 0.7-µ size fraction, whereas the smallest particles exhibit an autotrophic signature distinct from both heterotrophy and phototrophy.
The presence of all three trophic states in an RAS with ambient light suggests a functionally redundant microbial community that is prepared for carbon uptake and remineralization under fluctuating temperature, light, and pH conditions.
Nitrification
Farmers know that nitrification, or the oxidation of nitrate and ammonia, is an integral microbial process in an RAS. An active nitrifying community ensures that toxic ammonia and nitrite are rapidly oxidized to nitrate, which is relatively harmless to shrimp.
Rate measurements suggest that both nitrification and denitrification potentially take place simultaneously in the Oceanic Institute systems during the later stages of the grow-out period. In unamended RAS water, a significant increase in nitrate concentration occurs after 24 hours of incubation under oxic conditions (Fig. 4). When RAS water was amended with a nitrification inhibitor, there was no significant change in nitrate concentration under oxic conditions.
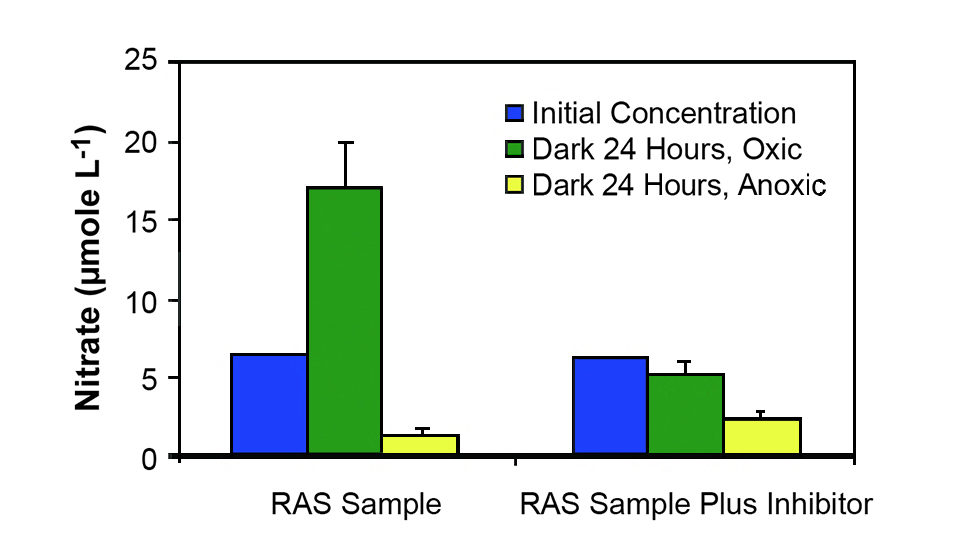
These results demonstrated nitrification in Ocean Institute systems. In addition, nitrate concentration decreased significantly under anoxic conditions, reflecting the potential impact of microbial denitrification on the removal of nitrogen from the RAS.
(Editor’s Note: This article was originally published in the June 2006 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Carolyn M. Holl, Ph.D.
Oceanic Institute
41-202 Kalaniana’ole Highway
Waimanalo, Hawaii 96795 USA[103,114,111,46,101,116,117,116,105,116,115,110,105,99,105,110,97,101,99,111,64,108,108,111,104,99]
-
Christine J. Tallamy
Oceanic Institute
41-202 Kalaniana’ole Highway
Waimanalo, Hawaii 96795 USA -
Shaun M. Moss, Ph.D.
Oceanic Institute
41-202 Kalaniana’ole Highway
Waimanalo, Hawaii 96795 USA
Tagged With
Related Posts

Intelligence
A brief look at genetically modified salmon
If approved by FDA, fast-growing genetically modified salmon will provide a safe and nutritious product similar to other farmed Atlantic salmon.

Intelligence
A land grab for salmon (and shrimp) in upstate New York
The operators of Hudson Valley Fish Farm see their inland locale as a pilot to prove that land-based fish farming, located in close proximity to major metropolitan markets, can be successful.

Responsibility
A look at unit processes in RAS systems
The ability to maintain adequate oxygen levels can be a limiting factor in carrying capacities for RAS. The amount of oxygen required is largely dictated by the feed rate and length of time waste solids remain within the systems.

Responsibility
A look at various intensive shrimp farming systems in Asia
The impact of diseases led some Asian shrimp farming countries to develop biofloc and recirculation aquaculture system (RAS) production technologies. Treating incoming water for culture operations and wastewater treatment are biosecurity measures for disease prevention and control.


