Through its Responsible Aquaculture Program, GAA has recommended a range of water quality standards for shrimp farm effluents. Part of a series, this article addresses pH.
Chemical concentrations often are expressed in gram molecular weights (moles, mol) of a substance per liter. In the case of hydrogen ions, concentrations usually are very small. For example, shrimp pond water can have a hydrogen ion concentration below 0.000000001 mol per liter.
In order to avoid using such small numbers, Danish chemist S. P. L. Sorensen suggested using the negative logarithm of the hydrogen ion concentration as an index. Called pH, this became the standard for expressing hydrogen ion concentration. Concentrations of 0.0000001 mol per liter and 0.000000001 mol per liter, which represent the usual hydrogen ion range in shrimp ponds, are more easily expressed as pH values of 7 and 9. Meters for measuring hydrogen ion concentration read directly in pH.
The pH scale
Water separates (dissociates) slightly into hydrogen ions (H+) and hydroxyl ions (OH-). In pure water, the amounts of H+ and OH- are equal, and its pH is 7 on a scale of 0 to 14. When there is more H+ than OH-, the pH of water will decrease below 7, and the water is said to be acidic. Conversely, when there is more OH- than H+, the pH rises, and the water is called basic or alkaline.
Desired pH
pH has an influence on living things. For most aquatic organisms, death will occur when pH falls below 4, or rises above 10 and remains there for an hour or more. Although aquatic organisms tolerate a wide range in pH, most do best at a pH between 6 and 9 (Table 1). Shrimp farmers should strive to maintain a pH between 6 and 9 in ponds, with the best pH range 7 to 8.5.
Boyd, Effect of pH on aquatic organisms, Table 1
| pH | Response |
|---|
pH | Response |
|---|---|
| 4 | Acid death point |
| 4-5 | Acid stress, no reproduction, slow growth |
| 5-6 | Acid stress, reproduction may be less than normal, slow growth |
| 6-9 | Best range for growth and reproduction |
| 9-11 | Alkaline stress, reproduction and growth adversely affected |
| 11 | Alkaline death point |
Effluents and pH standards
The reason for setting target pH standards of 6 to 9 for effluents is obvious. If shrimp farm effluents have a pH outside this range, adverse impacts resulting from acid stress of aquatic organisms may occur in the natural waters receiving pond effluents.
Factors influencing pH
The pH of shrimp pond water is influenced by many factors, including the pH of source water, pH and acidity of bottom soil, shrimp culture inputs, and biological activity. The most common cause of low pH in water is acidic bottom soils. Where pond soils are highly acidic, liming can be used to reduce soil acidity and increase the pH of the water. The application of burnt lime (calcium oxide) or hydrated lime (calcium hydroxide), however, can raise pH to unacceptable levels of 9.5 or even higher.
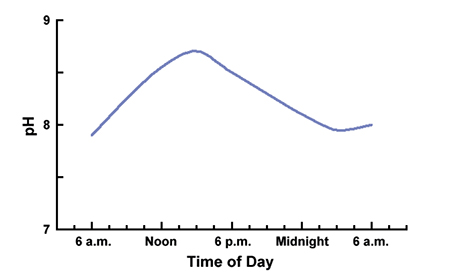
The most common cause of high pH is high rates of photosynthesis by dense phytoplankton blooms. In the daytime, phytoplankton remove carbon dioxide from the water for use in photosynthesis faster than carbon dioxide is released to the water by respiration. Because carbon dioxide is acidic, its net removal causes an increase in pH.
Pond waters typically increase in pH during the day and decrease in pH during the night (Fig. 1). It is not uncommon for the pH of surface water to increase to 9.5 or 10 during dense afternoon phytoplankton blooms. Of course, photosynthesis rate decreases with depth as light intensity decreases, so the pH near the pond bottom may be considerably lower than at the surface.
Measuring pH reliably
The only reliable way of measuring pH is with an electronic pH meter (Fig. 2). Portable meters measure pH in the field, or samples may be returned to a laboratory for measurement.
pH changes quickly in a water sample, so measurements should be made within 30 to 60 minutes of taking the sample. The sample should not be agitated or exposed to sunlight during the brief storage period. Measurements made in the early morning (6 to 8 a.m.) and in early afternoon (1 to 3 p.m.) will indicate the daily range in pH.
Standardization

A pH meter must be standardized before making measurements. Although it is customary to use a pH 7 buffer to standardize a pH meter, this procedure is not always reliable, for almost any pH meter can be adjusted to pH 7.0. The meter can be standardized at pH 7, but a second pH buffer one or two pH units above or below pH 7 should also be used to verify the meter will read another pH correctly after being standardized.
Few pH meters will read accurately over a wide pH range beyond the pH at which they were standardized. Thus, more accurate results will be obtained if the pH meter is standardized at a pH near the sample pH. For example, if samples are expected to have a pH of 8.5 to 9.5, it would be better to standardize the meter with a pH 9 buffer than a pH 7 buffer.
pH management
Water pH is an important variable to aquafarmers. For example, a water pH of 6 suggests that shrimp ponds urgently need to be limed.
High pH is much more difficult to bring into proper range than low pH. Farmers’ main control on high pH is to regulate fertilization, stocking, and feeding to prevent excessive phytoplankton blooms. When pH is high where water exchange is used, water can be discharged at night when pH is lower, or from near the pond bottom, where pH is lower than at the surface.
(Editor’s Note: This article was originally published in the February 2001 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Author
-

Claude E. Boyd, Ph.D.
Professor, Department of Fisheries and Allied Aquacultures
International Center for Aquaculture and Aquatic Environments
Auburn University
Auburn, AL 36849 USA
Tagged With
Related Posts

Responsibility
Constantly changing pH unavoidable, completely normal
Prof. Claude Boyd discusses the importance of pH for farmed fish and shellfish, the normal and natural fluctuations and how aquaculture systems can manage it.

Health & Welfare
Manage pH cycles to maintain animal health
The ideal pH for most aquaculture species is between 6.0 and 8.5. Lower pH values may result in decreased growth and survival, and greater susceptibility to disease. pH typically is lowest in the early morning, increases during the afternoon and declines at night.

Responsibility
Phosphates, pH management control algal blooms in barramundi ponds
Pond pH had a strong effect on the effectiveness of a product applied to remove excess phosphate to control algal blooms in eutrophic aquaculture ponds.

Health & Welfare
pH field measurements improve catfish fry survival
Since catfish fry survival lowers if subjected to an abrupt pH rise of 0.5, the authors recommend measuring pH in transport tanks and receiving ponds and the addition of buffering agents to transport tanks.

