Through its Responsible Aquaculture Program, GAA has recommended a range of water quality standards for shrimp farm effluents. Part of a series, this article addresses Total Ammonia Nitrogen (TAN).
Nitrogen is a key factor regulating productivity of natural and agricultural ecosystems because it is an essential plant nutrient, a major component of protein, and often in short supply. Nitrogen occurs with phosphorus in effluents, and these two nutrients are the major cause of eutrophication of water bodies.
Nitrogen cycle
A complex nitrogen cycle functions on a global basis. Most pathways within the cycle (Fig. 1) can be found in all ecosystems, including aquaculture ponds.
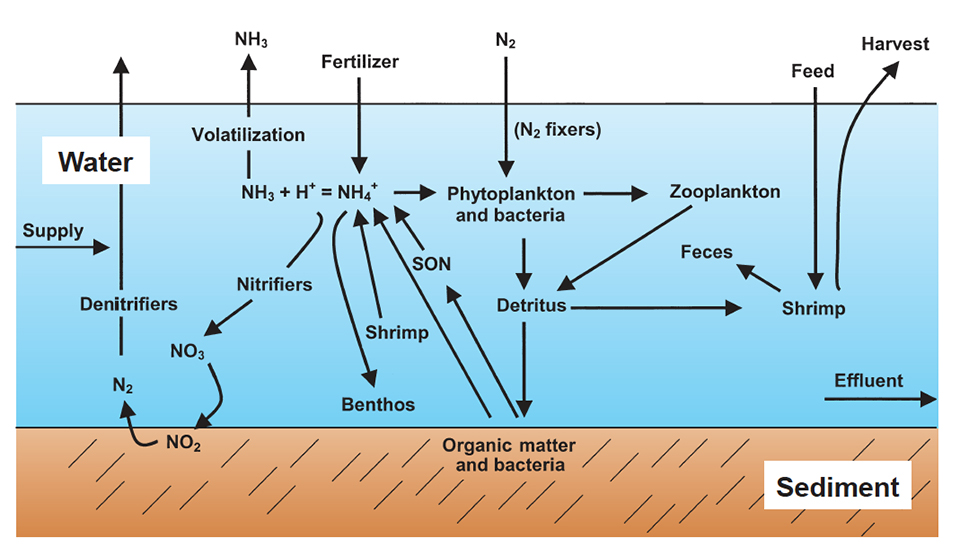
Vast amounts of nitrogen exist in the atmosphere in gaseous form. This nitrogen can be converted to nitrate by lightning, fixed in organic matter by some microorganisms, and converted to nitrogen for fertilizers by an industrial process. Large quantities of nitrogen are found in living organisms and dead organic matter.
Organisms excrete nitrogenous wastes, and when bacteria and other microorganisms decompose dead organic matter, they release ammonia nitrogen into the environment. Ammonia nitrogen consists of non-ionized ammonia (NH3) and ammonium ion (NH4 +). Nitrate nitrogen is produced when nitrifying organisms oxidize ammonia nitrogen.
Most plants prefer ammonium ion as a nitrogen source, but can also use nitrate as a nitrogen source. Nitrate can be converted back to nitrogen gas by the activity of denitrifying bacteria.
Use in aquaculture
Nitrogen is applied to aquaculture ponds in fertilizers such as urea, ammonium nitrate, and sodium nitrate to promote phytoplankton growth and enhance the production of the culture species. Feeds are commonly used in aquaculture because they allow greater production of the culture species than is possible with fertilizers alone.
Feeds usually contain 20 to 40 percent protein, and protein has an average nitrogen concentration of 16 percent. About 20 to 25 percent of the nitrogen applied to ponds in fertilizers and feeds is retained in the biomass of the culture species and removed from ponds at harvest (Table 1).
Boyd, Typical inputs and outputs of nitrogen, Table 1
| Inputs | Inputs | Outputs | Outputs |
|---|
Inputs | Inputs | Outputs | Outputs |
|---|---|---|---|
| Feed and fertilizer | 90% | Shrimp harvest | 25% |
| Water supply and nitrogen fixation | 10% | Denitrification | 12% |
| Ammonia volatilization | 18% | ||
| Sediment accumulation | 10% | ||
| Effluent | 35% |
Much of the fertilizer nitrogen is converted to organic nitrogen in plankton, and the culture species excretes ammonia nitrogen that also can be used by phytoplankton. Microorganisms decompose dead plankton, uneaten feed, and feces, and the nitrogen contained in these organic materials is recycled.
The two main forms of non-gaseous nitrogen in pond water are particulate and dissolved organic nitrogen and ammonia nitrogen. Nitrification occurs in aquaculture ponds, but much of the nitrate is denitrified within the sediment. Nitrate concentrations usually are much lower than those of ammonia nitrogen.
https://www.aquaculturealliance.org/advocate/water-quality-standards-total-phosphorus/
Ammonia in ponds
Ammonia nitrogen is of concern in pond effluents because it can cause eutrophication, waters with high ammonia nitrogen concentrations usually contain appreciable organic nitrogen, and ammonia nitrogen is potentially toxic to aquatic organisms. Nonionized ammonia and ammonium occur in water in a temperature- and pH-dependent equilibrium as follows:
NH3 + H+ = NH4+
As pH increases, nonionized ammonia increases. At pH 7 and 28 degrees-C, there is less than 1.0 percent nonionized ammonia, but at pH 9 and the same temperature, the proportion of the nonionized form increases to about 40 percent. Increasing temperature also causes the proportion of nonionized ammonia to rise, but temperature has a much smaller effect than pH.
Nonionized ammonia nitrogen is the toxic form of the compound. Concentrations of total ammonia nitrogen above 3 or 4 milligrams per liter can result in toxicity to warmwater aquatic organisms in waters with pH above 8.5 or 9.
Reducing eutrophication
By limiting the total ammonia nitrogen concentration in effluents to a concentration that will not cause toxicity in the mixing zone, the potential for eutrophication is also greatly reduced. Limits on total ammonia nitrogen commonly are established in effluent permits, but concentration limits for total nitrogen seldom are specified. Of course, in some permits, there may be a limit on total nitrogen loads.
The calculation of nitrogen load requires data on both effluent volume and total nitrogen concentration. For example, suppose the effluent volume for a shrimp farm is 20,000 cubic meters per day and the average total nitrogen concentration is 4 milligrams per liter (grams per cubic meters). The total nitrogen load is 20,000 cubic meters per day x 4 grams per cubic meter = 80 kilograms per day.
GAA effluent standards
The Global Aquaculture Alliance effluent standard for total ammonia nitrogen is initially 5 milligrams per liter, with a target of 3 milligrams per liter. Compliance with these standards is easy in semi-intensive aquaculture, where feed inputs are usually less than 30-40 kilograms per hectare per day. Total ammonia nitrogen concentrations seldom rise above 1 milligrams per liter and are rarely greater than 2 milligrams per liter. Semi-intensive farm managers strive to maintain concentrations below 1 milligrams per liter.
Because of the difficulties associated with setting different standards for different production levels, GAA adopted a total ammonia nitrogen standard based on intensive shrimp culture, where concentrations often exceed 5 milligrams per liter and can even reach 10milligrams per liter. Thus, compliance to GAA’s standard will be more difficult in intensive aquaculture.
Reducing ammonia in effluents
Methods for reducing total ammonia nitrogen concentrations in aquaculture pond effluents include the following:
- Use fertilizers in moderate amounts and only when needed to promote phytoplankton growth.
- Do not stock and feed at rates so high that water quality deteriorates in ponds.
- Avoid feeding more than culture species can consume.
- Purchase feeds that contain no more nitrogen than needed by the culture species.
- Use mechanical aeration in intensive ponds to avoid dissolved oxygen concentrations below 3-4 milligrams per liter.
Concentration changes in ponds
Total ammonia nitrogen concentrations in ponds change gradually over a period of days. They tend to increase as culture periods progress and feeding rates increase. Nonionized ammonia concentrations change daily as pH and water temperature increase and decrease in response to inputs of solar radiation.
The analytical methods generally used for ammonia nitrogen do not distinguish between non-ionized ammonia and ammonium. The non-ionized ammonia concentration must be estimated with a table that relates the percentage of non-ionized ammonia to temperature and pH. Such tables can be found in books on water quality in aquaculture.
It is sufficient to measure only total ammonia nitrogen in effluents to comply with GAA standards. Some effluent permits, however, require data on non-ionized ammonia.
Sampling and analysis
Samples for total ammonia nitrogen analyses should be placed in plastic bottles, kept on ice, and analyzed six to eight hours after collection. A private or university laboratory can be contracted to do the analysis, but it often will not be possible to send samples within the required time period. Thus, farm technicians can make the analyses.
For seawater or brackish water, the salicylate method is best for total ammonia nitrogen analysis. The Nesslers method and the phenate procedure are best suited for freshwater. The Hach Chemical Co. of Loveland, Colorado, USA sells total ammonia nitrogen test procedures for salicyate and Nesslers methods. Instructions for conventional laboratory measurement of total ammonia nitrogen by the three methods can be found in a standard water-analysis manual such as Standard Methods for the Examination of Water and Wastewater.
(Editor’s Note: This article was originally published in the August 2001 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Author
-

Claude E. Boyd, Ph.D.
Professor, Department of Fisheries and Allied Aquacultures
International Center for Aquaculture and Aquatic Environments
Auburn University
Auburn, AL 36849 USA
Related Posts

Responsibility
Water quality standards: pH
The pH of shrimp pond water is influenced by source water, pH and acidity of bottom soil, shrimp culture inputs and biological activity.

Responsibility
Correct liming improves pond water, bottom quality
Shrimp and fish farmers frequently apply liming materials to ponds to increase the pH of bottom soils, elevate alkalinity and water hardness and improve conditions for microbial activity and benthic animals.

Responsibility
Light penetration in water
Light penetrating water is scattered and absorbed exponentially as it passes downward. The presence of dissolved organic matter and suspended solids further impedes light penetration, and different types of solids absorb different wavelengths.

Health & Welfare
Evaluating commercial probiotic for juvenile Pacific white shrimp
This study evaluated the impact of feeding a commercial diet, top dressed with a commercial probiotic, to Pacific white shrimp in a biofloc-dominated tank system.

