Professor Boyd clears up confusion related to units of measure

Editor’s note: This article was updated on Nov. 27 to reflect a correction in the first equation. We apologize for the error.
Differences in the units (dimensions) by which water quality variables are reported can result in confusion and sometimes lead to faulty assessments. Issues related to units of measure for some common water quality values in aquaculture will be discussed.
The most common way of reporting concentrations of several water quality variables is to give them in milligrams per liter (mg/L) or micrograms per liter (mg/L). For example, dissolved oxygen, total alkalinity, and total ammonia nitrogen concentrations usually are in milligrams per liter, while trace metal concentrations are given more often in micrograms per liter. A concentration of 1 mg/L is equivalent to 1 part per million (1 ppm) as shown below:
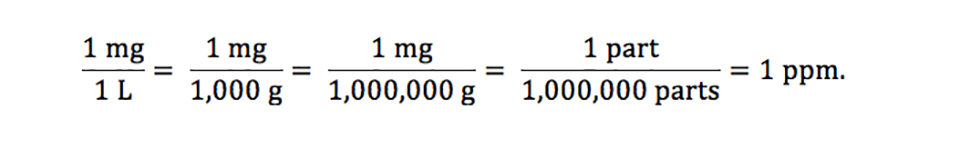
The reasoning can be extended to micrograms per liter, because 1 liter equals 1,000,000,000 mg and 1 u-g/L is equivalent to 1 part per billion (ppb). Milligrams and micrograms per liter are used interchangeably with parts per million and parts per billion, respectively.
To be completely correct, 1 mg/L and 1 mg/L equal 1 ppm and 1 ppb; respectively, only at 3.98 degrees-C. This is because 1 L of water weighs exactly 1.000 kg only at this temperature. In aquaculture water quality assessments, no serious error will be incurred by assuming 1 mg/L or 1 u-g/L equal 1 ppm or 1ppb, respectively, regardless of water temperature.
Inorganic substances
Concentrations of some inorganic substances such as ammonia, ammonium, nitrate, nitrite, and phosphate may be expressed as the concentration of the substance, e.g., as nitrite, or as the concentration of nitrogen, phosphorus, or other element in the substance, e.g., nitrogen in nitrate. It follows that 1 mg/L of nitrite is not the same as 1 mg/L of nitrite-nitrogen. The nitrite ion (NO2–) has a molecular weight of 46, while nitrogen’s atomic weight is 14, and 1 mg/L nitrite represents 0.304 mg/L nitrite-nitrogen (14/46 X 1 mg/L). On the other hand, 1 mg/L of nitrite-nitrogen would be equivalent to 3.29 mg/L nitrite (46/14 X 1 mg/L). There can be a huge error (about 10-fold too low or too high) from a misunderstanding of the way nitrite concentration is reported. Factors for converting between concentrations of the substance and the amount of the element in the substance for nitrate, nitrite, ammonia, ammonium, phosphate, and sulfate are presented (Table 1).
Boyd, Water Quality Variables, Table 1
| Substance | Formula of substance | Element in substance | Factor |
|---|---|---|---|
| Nitrate | NO3 | NO3- -N | 4.426 |
| Nitrite | NO2 | NO2- -N | 3.280 |
| Ammonia | NH3 | NH3-N | 1.216 |
| Ammonium | NH4 | NH4-N | 1.288 |
| Phosphate | PO4 | PO4-P | 3.067 |
| Sulfate | SO4 | SO4-S | 2.996 |
1Divide substance concentration (NO3, NO2, etc.) by factor to obtain concentration of element (N, P, or S) included in the substance. Multiply concentration as element in substance (NO3- -N, NO2- -N, etc.) by factor to obtain concentration of the substance (NO3, NO2, etc.).
Aquaculturists often use water quality test kits for measuring concentrations of water quality variables, and depending on the brand of test kit, either method of reporting concentrations mentioned in the paragraph above may be used. Some kits do not clearly specify the method, e.g., the kit may be called a nitrite test kit, but actually read out concentrations as nitrite-nitrogen.
Electrical conductivity
Units for electrical conductivity (specific conductance) also can be confusing. Traditionally, electrical conductivity was reported as the reciprocal of resistance which is measured in ohms. To avoid calling the unit of conductivity measurement reciprocal ohms – an awkward expression – ohms was spelled backwards giving mhos. The distance between electrodes in conductivity probes for water is 1 centimeter. The unit mhos per centimeter (mhos/cm) resulted, but to avoid small decimal fractions, meters usually readout in micromhos per centimeter (u-mhos/cm).
During the past two decades, many manufacturers have adopted the International System of Units (SI units) for conductivity meter readouts. Meters typically have functions to readout in either micro-Siemens per centimeter (mS/cm) or milli-Siemens per centimeter (mS/cm). One Siemens is equal to one mho; thus, 1 mS/cm equal 1u-mho/cm, and 1 mS/cm equals 1,000 u-mhos/cm.
Turbidity
The traditional unit of turbidity was the Jackson turbidity unit (JTU). This unit was based on the depth of water in a tube with a clear glass bottom required to obscure the flame of a candle positioned beneath the tube. The standard was a specific concentration of silica. Modern day turbidity determinations are made with a nephelometer that measures the amount of light reflected at 90 degrees from light beam passed through the water sample. The standard for turbidity by nephelometry is a colloidal suspension known as formazin, and the unit of measure is the nephelometer turbidity unit (NTU) or its equivalent, the formazin turbidity unit (FTU). There is no consistent correlation between Jackson turbidity units and formazin turbidity units. Turbidity in JTU cannot be compared with turbidity in NTU or FTU, and vice versa.
Salinity, hardness and alkalinity
Salinity often is reported as part per thousand (ppt), but it also may be reported as percentage, grams per liter (g/L), and milligrams per liter. One part per thousand is the same as 1 g/L as illustrated below:
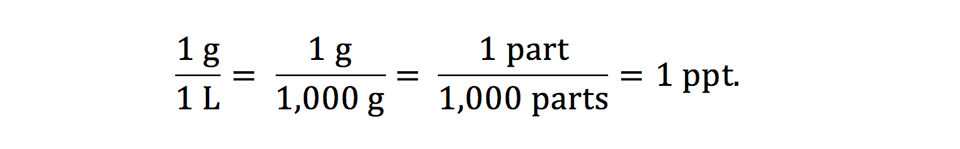
A salinity of 1 g/L is the same as 0.1 percent, 1 ppt, and 1,000 mg/L. Percentage is the same as one part in one hundred parts, and a 1 percent solution contains 10 ppt or 10,000 ppm (or mg/L).
Some water analysis kits report water hardness in grains per gallon. The unit, grain, was defined in antiquity as the weight of a grain of barley seed and used as a weight standard. Eventually, 1.0 grain was assigned a weight of 64.8 mg. One gallon contains 3.785 L, and 1.0 grain/L is equal to roughly 17.1 mg/L.
Hardness and alkalinity in water usually are expressed in milligrams of calcium carbonate (CaCO3). In most waters, hardness results from calcium and magnesium and alkalinity is mainly from bicarbonate. The calcium and magnesium concentrations can be multiplied by 2.5 and 4.12, respectively, to convert them to hardness equivalents (as CaCO3). The bicarbonate concentration multiplied by 0.82 is equivalent to alkalinity in waters with pH below 8.3.
Dissolved oxygen
Some modern dissolved oxygen meters have an option for measuring percentage oxygen saturation. Percentage saturation is the ratio of the measured dissolved oxygen concentration to the dissolved oxygen concentration at saturation multiplied by 100. For example, the measured dissolved oxygen concentration in freshwater might be 4.04 mg/L at 20 degrees-C at which the saturation concentration is 9.08 mg/L. The percentage saturation is 4.54 mg/L divided by 9.08 mg/L X 100 = 50 percent. Fish and shrimp actually respond to percentage saturation rather than to milligrams per liter, but aquaculturists are familiar with milligrams per liter or parts per million of dissolved oxygen. Most of the information on response of culture species was developed using milligrams per liter for concentration, and it is unwise to promote changing from milligrams per liter or parts per million of dissolved oxygen to percentage saturation of dissolved oxygen.
Author
-

Claude E. Boyd, Ph.D.
School of Fisheries, Aquaculture and Aquatic Sciences
Auburn University
Auburn, Alabama 36849 USA
Tagged With
Related Posts

Responsibility
Calcium and magnesium use in aquaculture
Aquatic plants and animals get the essential nutrients calcium and magnesium from water and food. Calcium concentrations impact the hydration and development of eggs in a hatchery, where calcium carbonate precipitation can be troublesome.

Responsibility
Electrical conductivity of water, part 2
The electrical conductivity of water is frequently measured in aquaculture systems and taken as an indicator of the degree of mineralization (total ion concentration) of water.

Responsibility
Does Coriolis force impact aerator placement in aquaculture ponds?
The Coriolis effect has no bearing on aerator placement and aquaculture pond management. The most important consideration with mechanical aeration is to provide a sufficient amount to maintain adequate dissolved oxygen concentration.

Health & Welfare
Acclimating shrimp postlarvae before pond stocking
Shrimp postlarvae acclimation before stocking into the various growout systems (ponds, raceways, tanks) is a critical – and often overlooked, sometimes taken for granted – step in the shrimp culture process. Various water quality parameters should be changed slowly so that the young shrimp have the time to gradually adapt to the new conditions.

