Histopathology, gene probes, electron microscopy and PCR are other techniques

at harvest.
In shrimp, the hepatopancreas is an organ of vital importance for the production of digestive enzymes, assimilation and storage of digestive products, delivery of nutrients to and removal of waste metabolites from the hemolymph, maintenance of salt balance and detoxification of heavy metals and foreign organic substances.
Necrotizing hepatopancreatitis bacterium (NHPB) is a pathogen mainly found in the tubular epithelial cells of the hepatopancreas, where it causes atrophy and malfunction. Shrimp digestion, lipid storage, healthy growth and development are impaired in infected animals.
NHPB affect juvenile and adult shrimp by the ingestion of infected material or cannibalism. Clinical signs of shrimp with necrotizing hepatopancreatitis (NHP) include soft and loose shells, flaccid bodies and dark gills. Animals become lethargic and anorexic. Slow growth, heterogeneous sizes at harvest, reduced feed consumption, low survival and poor feed conversion are registered in affected ponds, causing severe production and economic losses.
NHPB detection
NHP is believed to be associated with elevated water temperature and salinity based on an outbreak in Texas, USA, that occurred after temperatures rose over 29 degrees-C with salinities ranging from 20 to 40 ppt. These same environmental conditions are commonly found in northeastern Brazil’s shrimp farms throughout the year, where serious NHP outbreaks have also been reported. In the authors’ experience, the occurrence of NHP in semi-intensive pond systems is also highly associated with inadequate pond bottom management.
NHPB is a gram-negative, pleomorphic, rickettsialike intracellular bacterium belonging to the a-proteobacteria group, which cannot be cultured by traditional microbiological techniques. There is still a great deal of discussion among farm managers and researchers on the most effective way to diagnose this infection in situ.
Several techniques are claimed useful in detecting NHP, including wet mount analysis, histopathology, gene probes, electron microscopy and polymerase chain reaction (PCR). Among these, wet mount analysis has been the most used approach in the field, not only for health-monitoring programs but also for presumptive diagnoses. In Brazilian shrimp culture, it has been a common field practice to rely solely on wet mount observations to diagnose NHP in ponds and thereafter apply therapeutic treatments.
Testing methods compared
In a study, the authors compared the techniques of wet mount microscopy, histopathology and PCR in the diagnosis of NHP in farmed Litopenaeus vannamei and evaluated the usefulness of the wet mount technique as a field method for the diagnosis of this disease.
Shrimp of 10- to 11-g weight were randomly sampled with a cast net from three growout ponds at a semi-intensive commercial farm with reportedly high mortality caused by NHP in Rio Grande do Norte, Brazil. The hepatopancreas of each shrimp was divided into three pieces. One piece was used for wet mount and the other two were fixed for posterior histopathology and PCR analyses.
For wet mount analyses, one part of each shrimp hepatopancreas was set onto a glass slide with a 3 percent saline solution and observed under a microscope. Each sample was classified for loose cells from tubular epithelium, melanization and alteration in normal fluid color (orange to brown).
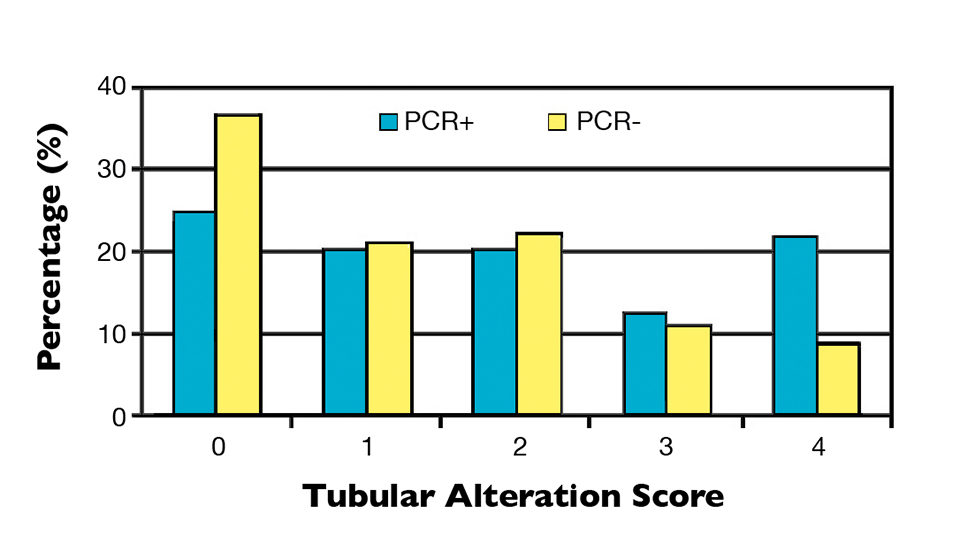
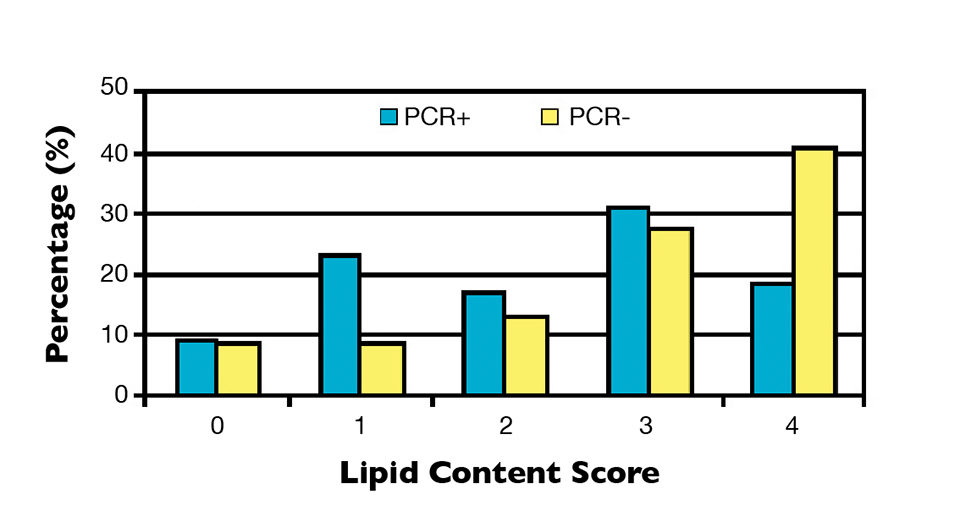
Tubular alteration and lipid content were classified into a score ranging from 0 (normal) to 4 (highly altered) and 0 (no lipids) to 4 (replete with lipids), respectively, which were directly related to shrimp nutritional status. Scores were allocated according to the severity of tubular alteration, based on the amount of alteration per field per animal, and quantity of lipids, based on the percentage of tubules filled with oil droplets per field per animal.
For histopathological analyses, samples were embedded with paraffin and then stained with hematoxylin and eosin. Each sample was classified depending on whether hemocytic infiltration, edema, granulomatous lesions and tubular atrophy were distinctly present.
Because of the sensitivity of detecting the presence of DNA of the causing agent in host shrimp, PCR was used as a control in this experiment to identify shrimp carrying NHP. PCR analyses were conducted following a well-known commercial protocol.
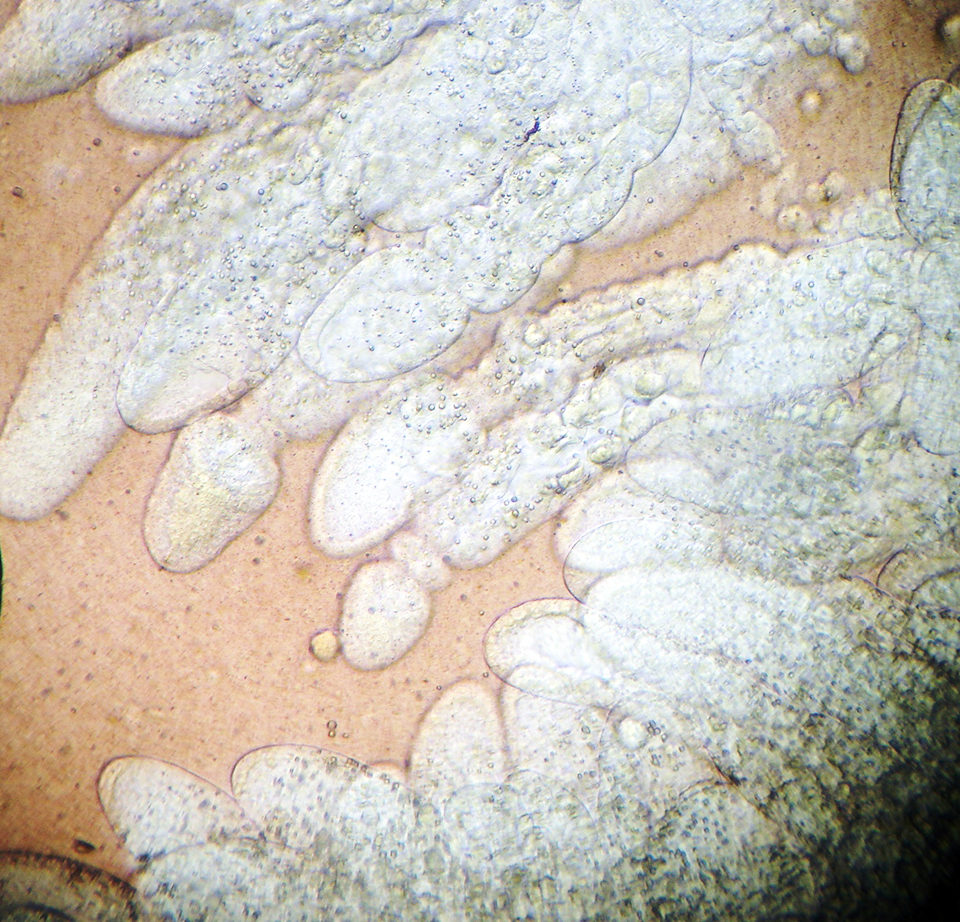
Results
For the hepatopancreas samples of 154 shrimp, 41.6 percent tested PCR positive and 58.4 percent tested PCR negative for NHPB. Infected animals scored lower lipid levels and higher tubular alteration, loose cells and melanization than those that registered as PCR negative. However, such differences were not statistically significant.
It was observed that 25 percent of NHP-infected samples possessed perfect hepatopancreas tubules (score 0), while 21.9 percent were severely altered (score 4). Although the PCR-negative group contained a higher percentage of individuals with normal tubules (36.7 percent), 42.2 percent of the individuals had a moderate, high or very high presence of severe alterations (Figure 1). Likewise with the tubular alterations, no clear relationship was observed between hepatopancreas lipid content and PCR results (Figure 2).
Histological analyses showed that both infected and uninfected shrimp had a high prevalence of hemocytic infiltration, edema and necrosis and atrophy in the tubular epithelium, with granulomatous lesions to a lesser extent (Table 1). The observed hepatopancreas characteristics or diagnosis criteria of the traditional approaches most correlated with PCR-positive results were that of lipid content (wet mount) and hemocytic infiltration (histopathology). However, in this study, a poor correlation (R = 0.07) was found among the PCR results and those parameters observed in wet mounts or histopathology, indicating that neither of these traditional techniques can be used alone to diagnose NHP.
Gomes, Mean percentages of histopathological lesions observed, Table 1
| Hemocytic Infiltration (%) | Hemocytic Infiltration (%) | Edema (%) | Granulomatous Lesion (%) | Tubular Atrophy (%) |
|---|
Hemocytic Infiltration (%) | Hemocytic Infiltration (%) | Edema (%) | Granulomatous Lesion (%) | Tubular Atrophy (%) |
|---|---|---|---|---|
| PCR+ (64) | 93.8 ± 24.4 | 54.7 ± 50.2 | 18.8 ± 39.3 | 51.6 ± 50.4 |
| PCR- (90) | 81.1 ± 39.4 | 57.8 ± 49.7 | 10.0 ± 30.2 | 52.2 ± 50.2 |
Perspectives
This work demonstrated that using the wet mount technique alone is inaccurate in the diagnosis of NHP because none of the observed lesions appeared to be disease specific, not to mention the subjective interpretation and grading by the examiner. The wet mount technique should be kept as a paramount tool for shrimp farmers to routinely check their stocks’ overall health and nutritional status. However, only PCR techniques should be considered for definite proof of the presence of NHP.
In fact, the hepatopancreas tubules of healthy shrimp typically show perfect fingerlike shapes replete with oil droplets, which is a good indicator of nutritional and overall health status and can be clearly observed in wet mount exams. Tubular alteration, melanization and low lipid levels in shrimp hepatopancreas tissue may be related to NHP, but can also be a consequence of factors such as quantity and quality of consumed feed, poor water or soil quality and environmental changes, and may also occur in shrimp infected with vibriosis, poisoned by toxins or with nutritional disorders.

It is important to note that environmental or pond management factors, rather than NHP, can play a significant role in results because uninfected animals also presented low lipid scores. In most disease-affected ponds, it is unlikely that a single etiology is the illness causative agent. The common case is to find more than one agent affecting either a single animal or an important part of the population.
Furthermore, the presence of several etiological agents or factors can lead to an inaccurate diagnosis, as viral infections are typically accompanied by secondary bacterial infections that can cause the death of animals previously weakened by a virus.
As with the wet mount examinations, none of the histopathological lesions were NHP-specific, because lesions can also be associated with other illnesses or toxins. For this reason, PCR is an important method for NHP diagnosis, as it allows the detection of non-culturable bacteria even when a large number of samples must be simultaneously analyzed.
A positive PCR result does not always mean that the pathogen is causing disease. Sometimes the host can coexist with the pathogen with little or no adverse effect as an overall function of host health and pathogen virulence, concentration and ability to compromise the host’s immunological defenses.
(Editor’s Note: This article was originally published in the November/December 2011 print edition of the Global Aquaculture Advocate.)
Authors
-
Giana Bastos Gomes
Aquaculture Genetics Research Group
School of Marine and Tropical Biology
James Cook University
Townsville, Queensland, Australia -
Jose Antonio S. Domingos
Aquaculture Genetics Research Group
School of Marine and Tropical Biology
James Cook University
Townsville, Queensland, Australia -
Veronica Arns Da Silva
Aquatic Organisms Health Laboratory
Universidade Federal Rural de Pernambuco
Dois Irmaos, Recife/P.E., Brazil -
Paulo De Paula Mendes
Aquatic Organisms Health Laboratory
Universidade Federal Rural de Pernambuco
Dois Irmaos, Recife/P.E., Brazil
Tagged With
Related Posts

Aquafeeds
A look at corn distillers dried grains with solubles
Corn distillers dried grains with solubles are an economical source of energy, protein and digestible phosphorus to reduce feed costs and fishmeal usage.

Aquafeeds
Animal co-product hydrolysates sources of key molecules in aquafeeds
Key molecules found in animal byproduct hydrolysates show potential for use as functional ingredients in aquaculture feeds. Animal co-product hydrolysates from slaughterhouse waste and rendered animal byproducts present a protein alternative.

Health & Welfare
Biofloc technology holds potential for carnivorous fish species
Juvenile carnivorous African catfish performed well in biofloc-based systems, which could help produce better quality and more disease-resistant seed of this important aquaculture species and support the expansion of African catfish farming industry.

Innovation & Investment
Connecticut ‘urban fish farm’ leans on RAS to produce branzino
The Ideal Fish “urban fish farm” in Connecticut is producing branzino, or European sea bass, using RAS technology. Learn about this project from the company that designed and supported it: Pentair Aquatic Eco-Systems.


