The main technique used to encourage diatoms is silicate fertilization

Silicon is second only to oxygen in abundance in the earth’s crust. Much sand consists of silica (silicon dioxide or SiO2), and clay minerals are hydrous aluminum silicates. Natural waters contain silicon because of the dissolution of silicate minerals with which they come in contact. For example, silicon dioxide reacts in water to form silicic acid, a weak acid that is largely un-ionized within the pH range of most natural waters. When calcium silicate reacts with carbon dioxide in water, the resulting dissolved substances are calcium ions, bicarbonate ions (alkalinity) and silicic acid.
Silicon concentrations in natural waters typically are reported in terms of SiO2 and usually range from 5 to 25 mg/L in freshwater bodies. The global average for silica in river water is 13.1 mg/L. Normal seawater contains 6.4 mg/L silica. A silica concentration can be converted to silicon concentration by multiplying by the factor 0.467, the proportion of silicon in SiO2.
Plants take up silicic acid from water. Silicon in higher plants is incorporated into cell walls, making stems and leaves more rigid and strong. Among the phytoplankton, diatoms have a particular need for silicon, because their frustules – the hard but porous cell walls – are composed almost entirely of silica. Depending upon the species, plants contain from less than 0.1 percent to as much as 10.0 percent silicon on a dry-weight basis. Diatoms contain the greatest amount of silicon.
Diatoms and silicon
The ratio of carbon:nitrogen:silicon:phosphorus in diatom cells averages 106:15:16:1. Thus, diatoms require about the same amounts of nitrogen and silicon for growth. There is evidence that nitrogen:silicon ratios above 3:1 lessen the growth rate of diatoms. There also is an opinion that diatoms increase in abundance in proportion to other planktonic algae as the nitrogen:phosphorus ratio in water widens. This hypothesis has not been proven in research, and moreover, planktonic algae – including diatoms – tend to have a nitrogen:phosphorus ratio of around 15:1.
Unpolluted seawater typically has a greater concentration of nitrate nitrogen than of ammonium nitrogen. Because diatoms thrive in seawater, they often are thought to prefer nitrate over ammonium as a nitrogen source. In addition, ammonium tends to diminish nitrate uptake by phytoplankton, and ammonium is the preferred nitrogen source of green and blue-green algae. Aquaculture ponds typically have higher concentrations of ammonium nitrogen than of nitrate nitrogen, a factor that would seem to discourage diatom growth and favor the growth of green and blue-green algae.
Studies of temperate-zone lakes have shown that diatoms often bloom in the spring, but this phenomenon leads to reduced silica concentration. Once the silica concentration declines, other types of phytoplankton that do not need silicon typically replace diatoms as the predominant form of planktonic algae.
Use of sodium nitrate and sodium silicate fertilizers at a 1:1 nitrogen:silicon ratio in water of 9 to 10 ppt salinity and containing 0.21 mg/L silica at the Claude Peteet Mariculture Center in Gulf Shores, Alabama, USA, was shown to be quite effective in increasing both the abundance of diatoms and their proportion of the total phytoplankton. Of course, in this situation, the concentration of silica was very low – normal seawater contains 6.4 mg/L silica. Diatoms grow quite well at the silica concentration in seawater, and it is not known how low the silica concentration must fall before diatom growth is negatively affected.
Promoting diatom production
The discussion above suggests a relationship among relative abundance of diatoms, nitrogen:silicon ratios, silicon concentration and nitrate availability. Diatoms are considered desirable in aquaculture ponds because they rarely are associated with water quality deterioration and are of good nutritional value to many aquaculture species and postlarval shrimp, in particular. Although there has been little effort to encourage diatom growth in freshwater aquaculture, shrimp farmers – especially in Central and South America – often attempt to increase the abundance of diatoms relative to other types of planktonic algae in marine and brackish water.
The main technique used to encourage diatoms is silicate fertilization, but sodium nitrate fertilization also is a common practice. Silicate fertilizers are used with little consideration of silica concentrations in ponds, and it seems unlikely that ponds with silica concentrations near that of normal seawater would respond to silicate fertilization. The prudent approach would be to measure silica concentration, and if it is less than the 6.4 mg SiO2/L value for seawater, silicate could be applied in an effort to obtain the target concentration.
It would be desirable to know the relationship between silica concentration and diatom growth, but this topic has not been investigated in aquaculture ponds. Lack of information about the relationships between silicate fertilization rates and silica concentrations in water, and how fast silica disappears from water also complicates efforts to encourage diatom growth.
Nevertheless, assuming complete dissolution of silicate fertilizers, 1 mg/L SiO2 would require 2.03 mg/L of sodium metasilicate or 1.93 mg/L of calcium metasilicate. In a 1-m-deep, 1-ha pond, the treatment rates necessary to obtain 1 mg/L of SiO2 would be 20.3 and 19.3 mg/L, respectively, for sodium metasilicate and calcium metasilicate. Silicate fertilizers do not dissolve completely when broadcast over ponds, and 1.5 to 2.0 times the rates estimated above should be used to increase silica concentration by 1 mg SiO2/L.
Silicate fertilizers
Several vendors offer standard fertilizers that contain 2.5 to 5.0 percent SiO2. At typical application rates of 10-20 kg/ha, the silica addition would not appreciably increase the dissolved silica concentration. Thus, farmers should use a silica product that contains 20.0 percent silicon (about 43.0 percent SiO2) rather than relying on the small amounts of silica often included in standard fertilizers.
Silicate fertilizers also are liming materials that can neutralize bottom soil acidity and increase total alkalinity in pond water. With calcium silicate, for example, the calcium ions applied to acidic sediment replace acidic ions in soil. Hydrogen ions resulting from displacement of acidic ions from soil are tied up in undissociated silicic acid:
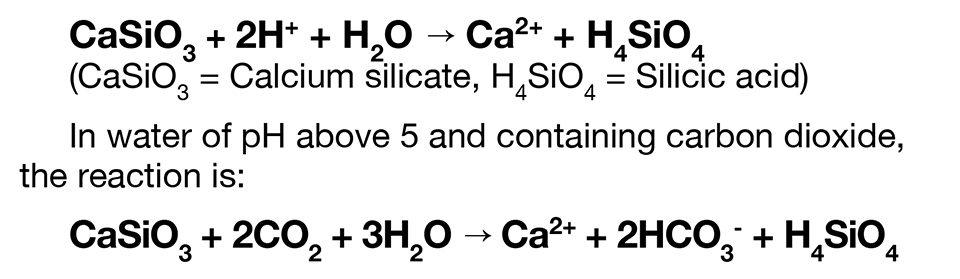
However, silicates have lower neutralizing values than agricultural limestone or lime, as illustrated in Table 1 for pure compounds. Agricultural limestone and lime are more readily available in most locations and also cheaper than silicates.
Boyd, Neutralizing values of pure compounds,Table 1
| Compound | Neutralizing Value (%) |
|---|---|
| Calcium silicate | 86 |
| Sodium silicate | 82 |
| Calcitic limestone (agricultural limestone) | 100 |
| Calcium hydroxide (hydrated lime) | 135 |
| Calcium oxide (burnt lime) | 179 |
(Editor’s Note: This article was originally published in the May/June 2014 print edition of the Global Aquaculture Advocate.)
Author
-

Claude E. Boyd, Ph.D.
Department of Fisheries
and Allied Aquacultures
Auburn University
Auburn, Alabama 36849 USA[117,100,101,46,110,114,117,98,117,97,64,49,101,99,100,121,111,98]
Tagged With
Related Posts

Aquafeeds
A new nutrient for aquaculture, from microbes that consume carbon waste
Biotechnology firm NovoNutrients aims to produce a line of nutraceutical aquafeed additives as well as a bulk feed ingredient that can supplement fishmeal. Its process includes feeding carbon dioxide from industrial gas to a “microbial consortium” starring hydrogen-oxidizing bacteria.

Responsibility
Inorganic fertilization of production ponds
Nitrogen and phosphorus are the most important nutrients in fertilization of both freshwater and coastal ponds. Many factors that can affect the response of ponds to fertilizers are location-specific. Aquaculture pond managers will have to figure out the best procedure for a given location or even for an individual pond.

Responsibility
The importance of liming materials in aquaculture
Liming is an important management tool for preparing aquaculture ponds between and during production cycles. Several products are sometimes used in aquaculture for liming production ponds, including agricultural limestone, calcium silicate (CaSiO3) and sodium bicarbonate (NaHCO3).

Health & Welfare
How decomposition of organic matter impacts aquaculture ponds
Organic matter decomposition causes most of the water quality issues in aquaculture ponds. Bacterial populations are the primary organisms of decay in an aquaculture system, including its organic matter.

