Fast-growing fish could safely deliver healthy seafood

In the not-too-distant future, your customers may ask if your Atlantic salmon is genetically modified (GM). GM salmon have been in the news recently, although the product is not yet on the market. But when and if they are approved by the United States Food and Drug Administration (FDA), as recommended by its advisory committee, GM salmon will provide a safe, nutritious and delicious product similar to other farm-raised Atlantic salmon.
People generally fear what they do not understand – especially when it’s rumored to be dangerous to their health and the environment. AquAdvantage salmon provide a prime example of how a lack of knowledge can breed unfounded fears. Key facts about the salmon can help put such fears to rest.
AquAdvantage salmon
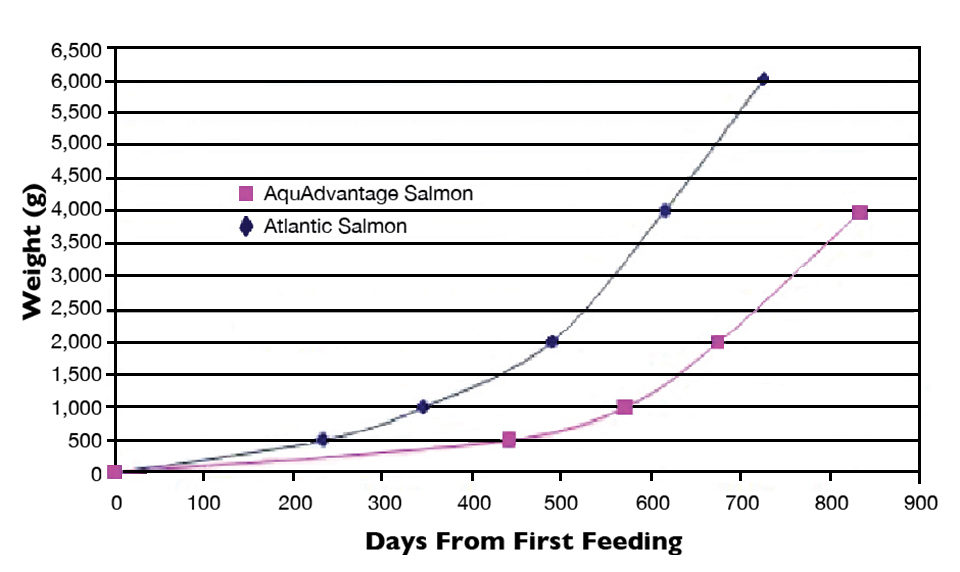
AquAdvantage Atlantic salmon have one added gene and a promoter that enhance their growth. One is a gene from a Chinook salmon that results in production of salmon growth hormone. This salmon gene is regulated by a segment of DNA from the ocean pout, a small eel-like marine fish found in frigid waters of the Northwest Atlantic.
AquaBounty Technologies, a company located in Massachusetts, USA, submitted an application to the FDA to grow the genetically enhanced fish on Prince Edward Island, ship small fish to inland recirculating systems in Panama, harvest and process the fish, and ship food-grade product back to the United States for sale.
Safety measures
Concerns have been raised that GM salmon could escape and breed in the wild, but the fish for this production system would be 95 percent triploid females as a double measure to prevent reproduction. Triploid crops have an extra set of genes that makes them sterile and unable to produce eggs or sperm. In agriculture, triploid crop examples include Gravenstein and MacIntosh apples; seedless bananas, watermelons and grapes; and microbial-resistant soybeans, papayas and tomatoes.
As an extra precaution against the risk of escaping and breeding, GM salmon will be grown in self-contained, land-based systems rather than in cages in open water. This is in line with the draft Best Aquaculture Practices certification standards for salmon farms, which do not permit the farming of transgenic fish in cages in open water.
FDA review process
The review process used by FDA for the AquAdvantage salmon involved the Veterinary Medicine Advisory Committee (VMAC), a group of government and non-government scientists who advise the commissioner of food and drugs regarding public and animal health. In September 2010, the VMAC published a 180-page scientific report that concluded AquAdvantage salmon was safe, nutritionally comparable to other Atlantic salmon and not a threat to the environment.
The review process used by FDA on the AquAdvantage salmon was more rigorous and extensive than that applied to any other food in history. Specific conclusions from the report included:
- Food from AquAdvantage salmon is the same as food from other Atlantic salmon.
- Food from AquAdvantage salmon is as safe to eat as food from other Atlantic salmon.
- AquAdvantage salmon meet the standard of identity for Atlantic salmon established by FDA’s Regulatory Fish Encyclopedia.
- Substantial, reliable information is available in the environmental assessment document to conclude that AquAdvantage Atlantic salmon are not expected to have a significant impact on the quality of the human environment in the United States, foreign nations not involved in the action or the global commons when raised and reared under the current conditions of physical, biological and geographical confinement present at the hatchery and growout facilities in Canada and Panama.
Healthy food choice
Farmed, wild and GM salmon are healthful food choices that are low in total fat and high in protein. All are rich in vitamins, minerals and omega-3 fatty acids. In recent years, research has linked seafood consumption to many health benefits throughout life.
Babies of mothers who eat fish during pregnancy have the best possible brain and eye development. Adults who eat fish twice a week have up to a 40 percent lower risk of dying from a heart attack. And a seafood-rich diet can help prevent depression and dementia as people age.
Healthy advances
New technologies to genetically improve food and animal crops are a tool to supply additional food and improve human health. They can reduce the use of pesticides and fertilizers, and shrink the carbon footprints of animal and plant agriculture. GM foods have been eaten by millions of people worldwide for 15 years with no reports of ill effects.
By taking time to learn the facts and understand the science, we can all appreciate new advances in our ability to produce healthy, sustainable food with the confidence that it has been found safe through rigorous scrutiny and the world’s best science.
(Editor’s Note: This article was originally published in the March/April 2011 print edition of the Global Aquaculture Advocate.)
Authors
-
Paul G. Olin, Ph.D.
University of California
Cooperative Extension
Sea Grant Extension Program
133 Aviation Boulevard, Suite 109
Santa Rosa, California 95403 USA -
Pamela D. Tom
Seafood Network Information Center
University of California
Food Science and Technology Department
Davis, California, USA
Related Posts

Intelligence
A land grab for salmon (and shrimp) in upstate New York
The operators of Hudson Valley Fish Farm see their inland locale as a pilot to prove that land-based fish farming, located in close proximity to major metropolitan markets, can be successful.

Innovation & Investment
Aquaculture Exchange: Ron Stotish, AquaBounty Technologies
Ron Stotish, CEO of AquaBounty Technologies, discusses being the first to produce a genetically modified (GM) farmed salmon deemed safe for consumption, the controversy surrounding his company's product and the potential of biotechnology.

Innovation & Investment
AquaBounty, with new RAS facility, hopes to win public support for GM salmon
Ron Stotish, CEO of AquaBounty Technologies, believes genetically modified salmon is no threat to its opponents and the outlook for AquAdvantage is good. With its purchase of the Bell Fish Co. RAS facility, commercialization will soon commence.

Intelligence
GM salmon and the FDA: 10 takeaways
After a 20-year process, a genetically modified fish earned U.S. Food and Drug Administration approval, reigniting one of the seafood industry’s most intriguing controversies. Here are 10 key downloads from the groundbreaking decision over AquAdvantage salmon.


