Carbon dioxide concentrations usually are highest when dissolved-oxygen concentrations are lowest

Carbon dioxide is both a nutrient and a waste product in aquaculture. Phytoplankton require carbon dioxide for photosynthesis. All organisms release carbon dioxide as a respiratory waste, and high concentrations of this metabolite can be harmful to aquatic animals. The concentration of carbon dioxide also is a major factor in controlling the pH of water.
Atmospheric carbon dioxide
The atmosphere is a vast reservoir of carbon dioxide. The average carbon dioxide concentration in the atmosphere is 380 ppm – up from 315 ppm in the 1950s and 280 ppm before the industrial revolution. Readers have no doubt heard concerns about the increasing atmospheric carbon dioxide concentration contributing to global warming.
Carbon dioxide is acidic, for it reacts with water to form carbonic acid. This acid dissociates into hydrogen ion and bicarbonate ion.
Rainfall becomes saturated with carbon dioxide while falling through the air. At the present atmospheric carbon dioxide concentration, the pH of rainfall normally should be around 5.6. Although rainfall is naturally acidic, there are regions where pollution of the atmosphere with sulfur dioxide and other wastes from combustion of fuels results in rainfall with much lower pH values that sometimes drop below 4.
Behavior in aquaculture systems
The carbon dioxide concentration at saturation in pure water is quite low (Table 1). Nevertheless, the small amount of carbon dioxide in rain enhances the dissolution of limestone and calcium silicate in soil and other geological formations to yield bicarbonate and calcium ion – sources of alkalinity and hardness, respectively.
Boyd, Solubility of carbon dioxide (mg/l) in pure water, Table 1
| Temperature (° C) | Salinity (ppt) 0 | Salinity (ppt) 10 | Salinity (ppt) 20 | Salinity (ppt) 30 | Salinity (ppt) 40 |
|---|---|---|---|---|---|
| 0 | 1.09 | 1.03 | 0.98 | 0.93 | 0.88 |
| 5 | 0.89 | 0.85 | 0.81 | 0.77 | 0.73 |
| 10 | 0.75 | 0.71 | 0.68 | 0.64 | 0.61 |
| 15 | 0.63 | 0.60 | 0.57 | 0.54 | 0.52 |
| 20 | 0.54 | 0.51 | 0.49 | 0.47 | 0.45 |
| 25 | 0.46 | 0.44 | 0.42 | 0.41 | 0.39 |
| 30 | 0.40 | 0.39 | 0.37 | 0.35 | 0.34 |
| 35 | 0.35 | 0.34 | 0.33 | 0.31 | 0.30 |
In a system prepared by placing calcitic limestone in distilled water and allowing it to equilibrate with the atmosphere, the total alkalinity would be about 58 mg/L, but in natural waters and aquaculture ponds, total alkalinity concentration can be greater. In areas with calcareous soils, alkalinity in aquaculture ponds can exceed 150 mg/L, and in arid regions, even higher values can occur. Seawater typically contains 142 mg/L alkalinity.
The concentration of plant-available inorganic carbon is much greater in a system where carbon dioxide is in equilibrium with calcium carbonate and dissolved bicarbonate than in pure water where the equilibrium is between atmospheric carbon dioxide and dissolved carbon dioxide only. When plants remove carbon dioxide from the water in natural systems and aquaculture ponds, more carbon dioxide is made available from the bicarbonate. To illustrate, at 25 degrees-C and pH 7, water with 50 mg/L total alkalinity would contain about 15 mg/L of available carbon. Pure water in equilibrium with air at 25 degrees-C would have less than 1 mg/L available carbon.
Application of liming materials to low-alkalinity waters to increase pH also increases the alkalinity and availability of inorganic carbon for plants. The dissolution of liming material and associated increase in alkalinity can be greatly enhanced by applying liming materials and organic matter to ponds simultaneously. Bacteria decompose the organic matter to increase the amount of carbon dioxide available to react with the liming material.
Relevance of phytoplankton
During daytime, phytoplankton normally remove carbon dioxide from water faster than it is released by respiration, causing a decline in carbon dioxide concentration and increase in pH (Fig. 1). In waters below 50 mg/l alkalinity, pH can rise to 9.5 or more in response to rapid photosynthesis. The pH rise usually is not as great in waters of 50 to 200 mg/l alkalinity because buffering by the alkalinity lessens the pH change. Waters of high alkalinity concentration often have a permanently high pH.
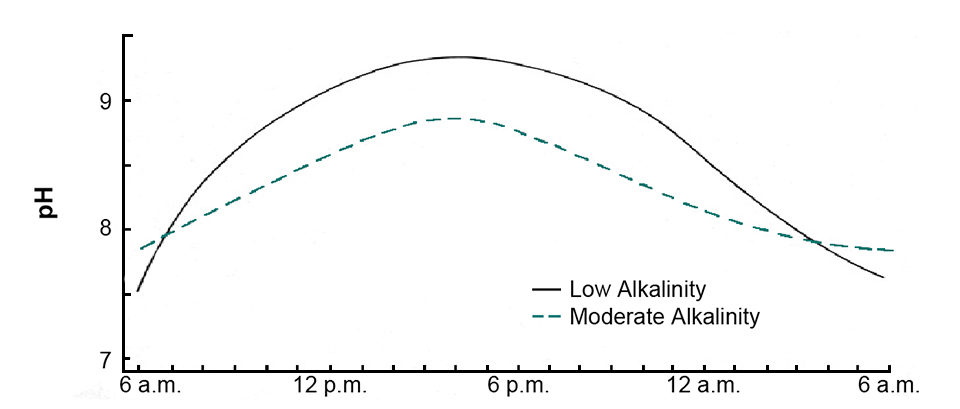
Phytoplankton use carbon dioxide as long as it is present, but when pH reaches 8.3, it is depleted. Most algal species can obtain carbon from bicarbonate when pH is above 8.3. The removal of carbon from bicarbonate by plants results in the release of carbonate ions into the water. Carbonate is alkaline and causes a further increase in pH. However, calcium reacts with carbonate to form calcium carbonate and limit the increase in pH.
Blue-green algae are more efficient than other types of algae in using low concentrations of carbon dioxide and obtaining carbon from bicarbonate. Thus, the abundance of blue-green algae often increases in nutrient-rich waters, where pH tends to increase over time because of the removal of inorganic carbon for photosynthesis.
Studies have revealed that blue-green algae often are replaced by other more desirable phytoplankton species when carbon dioxide is bubbled into the water column. Thus, some pond managers feel that application of organic matter to provide additional carbon dioxide can be beneficial in reducing pH and controlling blue-green algae.
Carbon dioxide concentrations
Carbon dioxide concentrations in aquaculture ponds typically are 5 to 10 mg/L in early morning, but can exceed 20 mg/L in ponds with large inputs of feed. Carbon dioxide concentrations usually are highest when dissolved-oxygen concentrations are lowest.
Carbon dioxide is not particularly toxic to fish and other aquatic animals, but fish generally avoid carbon dioxide concentrations above 5 to 10 mg/L. Concentrations of carbon dioxide above 20 mg/L are suspected to stress aquatic animals if exposure exceeds a few hours. Carbon dioxide at more than 60 mg/L can have a narcotic effect, and higher concentrations can cause death.
High concentrations of carbon dioxide in water interfere with carbon dioxide excretion through the gills of fish. This causes elevated concentrations of carbon dioxide in the blood plasma, which decreases blood pH. This condition lessens the ability of hemoglobin to bind oxygen and reduces oxygen uptake by blood at the gills, even when dissolved oxygen concentration is high.
The overall effect of elevated carbon dioxide concentration in water is to reduce respiratory efficiency and the tolerance of aquatic animals to low dissolved-oxygen concentrations. Thus, high carbon dioxide concentrations are particularly undesirable when dissolved-oxygen concentration is low – less than about 25 percent of saturation.
Mechanical aeration of pond water adds dissolved oxygen and removes carbon dioxide. Thus, the combination of low dissolved oxygen and elevated carbon dioxide concentration that can occur in early morning is especially troublesome where mechanical aeration cannot be applied.
Well water
Water that infiltrates downward through the soil becomes depleted of dissolved oxygen and supersaturated with carbon dioxide while passing through the root zone. Groundwater often has high carbon dioxide and low dissolved-oxygen concentrations. Where wells are used to supply ponds, this usually has no effect on culture species.
Hatchery waters should be aerated to add dissolved oxygen and remove carbon dioxide. It usually requires more aeration to saturate water with dissolved oxygen than to reduce carbon dioxide concentration to saturation. A high concentration of carbon dioxide in well water introduced into ponds usually does not have negative impacts on aquatic organisms.
CO2 removal
Carbon dioxide can be removed from water by treatment with lime, calcium oxide or calcium hydroxide. Thus, lime can be applied to ponds during crises with low dissolved oxygen to remove carbon dioxide and allow fish to use the existing dissolved oxygen more efficiently.
Based on chemical relationships, the removal of 1 mg/L carbon dioxide requires about 0.84 mg/L calcium hydroxide or 0.64 mg/L calcium oxide. However, lime does not dissolve quickly to react completely with carbon dioxide, and the theoretical rates mentioned above should be doubled.
(Editor’s Note: This article was originally published in the July/August 2008 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Author
-

Claude E. Boyd, Ph.D.
Department of Fisheries and Allied Aquacultures
Auburn University
Alabama 36849 USA[117,100,101,46,110,114,117,98,117,97,64,49,101,99,100,121,111,98]
Tagged With
Related Posts

Aquafeeds
A new nutrient for aquaculture, from microbes that consume carbon waste
Biotechnology firm NovoNutrients aims to produce a line of nutraceutical aquafeed additives as well as a bulk feed ingredient that can supplement fishmeal. Its process includes feeding carbon dioxide from industrial gas to a “microbial consortium” starring hydrogen-oxidizing bacteria.

Responsibility
Aquaculture ponds hold carbon
Although 16.6 million metric tons of carbon are annually buried in aquaculture ponds, estimated carbon emissions for culture species have approached several metric tons of carbon per metric ton of aquaculture product.

Responsibility
Assessing the carbon footprint of aquaculture
A carbon footprint is an estimate of the total carbon emissions resulting from the production, use and disposal of a product or service. Carbon footprints for aquaculture products result mainly from the use of manufactured feed and mechanical aeration.

Responsibility
Bacterial amendments in shrimp grow-out ponds
Pond microbial communities are a critical and often overlooked component of aquaculture ecosystems. Bacterial amendments like probiotics provide significant support to shrimp farmers around the world.

