Results indicate ozone could be recommended for water treatment in brackish water RAS for shrimp

Recirculating aquaculture systems (RAS) for shrimp culture are usually stocked with specific pathogen‐free (SPF) postlarvae (PL) to reduce the infection risk for shrimp, especially for viral and parasitic pathogens. Bacteria on the other hand are always present in shrimp RAS and are even necessary for the maintenance of optimal water conditions, including nitrifying and heterotrophic bacteria. The high stocking densities typically used results in large amounts of organic material from faeces, feed not consumed and low water exchange rates, so the load of heterotrophic bacteria can increase significantly in shrimp RAS when no management strategies are implemented for reduction of bacteria numbers.
Controlling potentially pathogenic, mostly heterotrophic bacteria is essential in RAS shrimp production and different approaches are used to reduce the risk of bacterial infections and to reduce the total amount of bacteria. The goal is a general reduction of high bacterial numbers in the water and the stabilization of a mature physiological microflora to prevent the establishment of potentially pathogenic bacteria within the mature microflora. For bacterial reduction, physical methods such as ozonation and ultraviolet (UV) irradiation are used, either separately or in combination
Any disinfection method may not only reduce pathogens but also can decimate beneficial microbial populations and favor the proliferation of opportunistic pathogens. Therefore, not only bacterial reduction but also the stabilization of the physiological microflora should be considered. Most studies conducted on disinfection of RAS waters have been for finfish production, and limited information is available for shrimp RAS
This article – adapted and summarized from the original publication [Teitge, F. 2020. Water disinfection by ozonation has advantages over UV irradiation in a brackish water recirculation aquaculture system for Pacific white shrimp (Litopenaeus vannamei). J. Fish Diseases 43(1): 1259-1285, October 2020.] – reports on a study that investigated the effect of different doses of UV light as well as the effect of ozone application on the microflora in RAS culture of L. vannamei.
https://www.aquaculturealliance.org/advocate/power-move-japanese-energy-firm-getting-in-on-ras-shrimp/
Study setup
This study was carried out at the Fish Disease Research Unit, University of Veterinary Medicine, Hannover, Germany, using six separate laboratory-scaled RAS. Each RAS had three holding tanks, a biofilter and a sedimentation tank. All RAS were maintained with the addition of food for three months before shrimp were introduced into the holding tanks. Water temperature was adjusted to 30 degrees-C and water salinity to 15 ppt.
For both experiments, specific pathogen‐free L. vannamei from a commercial hatchery (Shrimp Improvement Systems, Florida USA) were procured as PL 12 and acclimated for three weeks in a separated holding tank. After the acclimatization period, the shrimp were transferred to the holding tanks of the different RAS for each experiment. Automatic feeders were used in each tank, and before and after the start of UV irradiation and ozone application, the body weights and lengths of six shrimp per tank were recorded.
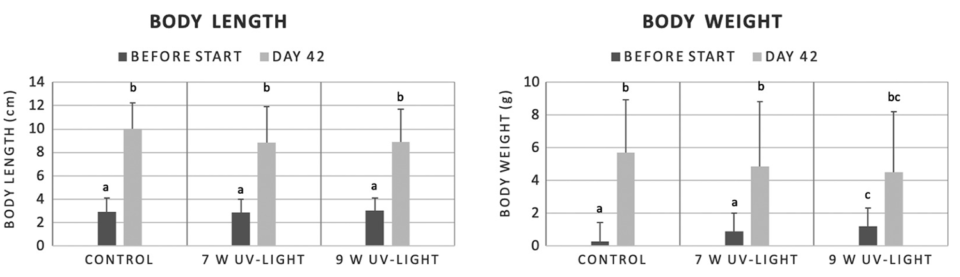
At the start of the UV irradiation experiment, the shrimp were approximately 39 days old with a mean body weight of 0.79 ± 0.46 grams and a mean body length of 2.94 ± 0.07 cm. Shrimp used in the ozone experiment were approximately 107 days old with a mean body weight of 5.56 ± 0.20 grams and a mean body length of 9.65 ± 0.09 cm. For both experiments the holding tanks of each RAS were stocked with 20 shrimp.
To assess the effect of UV irradiation and ozonization on the bacterial microflora in the RAS at each sampling timepoint, water samples, swabs from the biofilm of the tank surface and swabs from the transition from the carapace to the abdominal segments of three shrimp were collected from each holding tank. Swabs from the abdominal cavity of three shrimp per holding tank were also taken at the first and the last sampling timepoints.
For detailed information on the experimental design and RAS setup used; shrimp husbandry; UV irradiation and ozonation; bacteria culturing, identification and diversity; and statistical analyses, consult the original publication.
Results and discussion
We evaluated the effects of UV irradiation and ozone application in a brackish water RAS experimental setup for L. vannamei and investigated the bacterial compositions in the water, on the biofilms of tank surfaces and on shrimp carapaces and in the abdominal cavities of the animals. Additionally, the performance of the shrimp and the chemical water quality in the RAS were analyzed.
Ultraviolet irradiation and ozone application are widely used in aquaculture systems for finfish production mainly prophylactically for a general water disinfection with the aim of reducing the number of heterotrophic bacteria in the system and thus preventing the animals from disease outbreaks due to infections with facultative pathogens. Both methods are known for many years for their use in finfish aquaculture and even in shellfish facilities, studies on the effectiveness of UV irradiation were performed.
In general, the advantages of the use of UV irradiation over ozone application are lower costs and an easier maintenance of the reactors. Ozone application cost more and are also very complex. However, in RAS the costs can be lowered when other gases like oxygen are applied to a specific tank separated from the holding tanks, as only an ozone reactor has to be installed and because the water in RAS setups is reused, the amount of needed ozone is reduced as well
In our study, only slight differences in the performance of the shrimp were observed between both methods. In the UV irradiation experiment, the shrimp from the RAS treated with UV irradiation at a power of 9 watts gained slightly less body weight compared to the shrimp from the other treatment groups. However, these shrimp were significantly heavier at the start of the experiment compared to shrimp from the other treatment groups, so this has to be considered. In the ozone experiment, the shrimp were slightly shorter compared to those of the control group after 31 days.
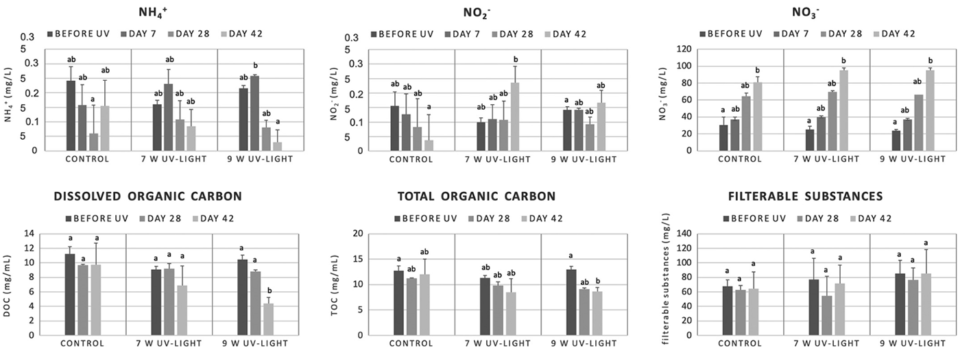
Regarding the chemical water quality, UV irradiation led to a reduced amount of ammonia in the water of RAS only when UV reactors with a power of 9 Watt were used, and no reducing effect was seen for the concentrations of nitrite and nitrate. Ozone application on the other hand prevented a nitrite increase and led to a faster decrease of nitrate compared to the control RAS. This benefit of ozone is widely known and makes ozone even more effective in RAS than UV irradiation
The main focus of our study was the influence of the two disinfection methods on the bacterial community composition in the water, on biofilms of tanks and shrimp carapaces and on the bacteria present in the abdominal cavity of the shrimp. The bacterial community was analyzed by culture techniques followed by molecular biological identification. Not all bacterial species present in the samples might be detected by cultivation methods because not all bacteria grow on agar plates, and this has to be considered when interpreting the data. However, the main bacterial species in aquaculture systems belong to the γ‐Proteobacteria and are culturable.
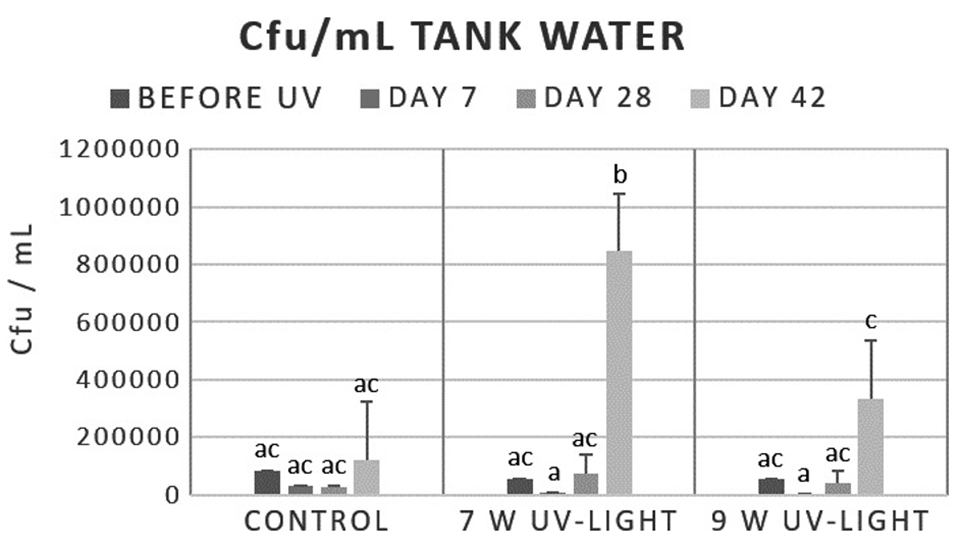
Our data showed that the total bacterial amount in the water could not be decreased significantly over time neither by UV irradiation nor by ozone application. After application of ozone the bacterial numbers in water stayed at a similar level during the complete experiment, but in the RAS treated with 7-watt UV light the total bacterial numbers increased until the end of the trial. Since practical conditions were to be tested in the present study, UV irradiation with a power recommended by the manufacturer for the given water volume was used. Using higher powers of UV irradiation could have achieved a greater reduction of the total bacteria numbers.
In our study, a reduction of Vibrio spp. could not be achieved by UV irradiation or ozone application. In general, the composition of bacterial genera stayed very stable and comparable to the control RAS in the ozone experiment and changed to a larger extent in the UV irradiation experiment. But after ozone application, we observed a shift in the detected Vibrio species: the number of V. alginolyticus – a potential pathogenic bacterium for shrimp that was detected in high numbers before the start of both experiments – decreased significantly over time after treatment with ozone and was not detected anymore on the carapaces of shrimp at the end of the experiment. Concurrently, the number of potentially pathogenic V. parahaemolyticus increased.
In our UV irradiation experiment, the number of V. alginolyticus decreased far less and was still highly abundant on day 42 in the biofilms of tank surfaces and on the carapaces of shrimp, especially in the RAS treated with 9-watt UV light. And the number of V. parahaemolyticus concurrently increased in this RAS. V. alginolyticus is known for forming biofilms that are able to survive UV treatments. Our results suggest that V. alginolyticus could be eliminated from the RAS by ozone but not by high dosage of UV irradiation. The reason for this different effect on V. alginolyticus was probably that a certain number of these bacteria was organized in biofilms and UV irradiation could only reduce bacteria in the circulating water. This underlines the statements from other, previous studies emphasizing that care must be used when determining the effective ozone or UV dose to achieve disinfection, because specific pathogens may require much higher doses for effective inactivation.
In our trials, the application of ozone showed advantages over disinfection by UV irradiation because the chemical water quality was improved and the microflora in the whole systems was more stable. Our previous studies on the microbial community in freshwater RAS had shown that a stable microflora appears to be very important to avoid the rapid growth of potentially pathogenic (to fish) bacteria. Biofilms can especially act as reservoirs for potentially pathogenic bacteria, and therefore, stable biofilms formed by harmless bacterial species, like we observed in the ozone trial, could reduce the risk of infections caused by opportunistic pathogens.
In general, the bacterial composition in the RAS changed to a greater extend after UV irradiation but stayed relatively stable and comparable to those of the untreated control RAS after ozone application. Differences in the microbial community in RAS can be due to disinfection but also to varying water exchange and reduced feed inputs. Therefore, general management strategies in RAS are at least as important as disinfection measures. We had previously shown that the combination of ozone application followed by UV irradiation was more effective compared to the use of only one method alone in reducing bacteria counts in freshwater aquaculture systems. We did not test the combined effect of UV irradiation and ozone application and suggest further studies in brackish water RAS for shrimp to evaluate these combined effects.
Perspectives
Based on the results of our study, the use of ozone in brackish water RAS for Pacific white shrimp has beneficial effects on chemical parameters and stabilizing effects in the microbial water quality. In contrast, UV irradiation was less effective in optimizing the chemical water quality and led to more significant changes in the microbial composition. This might have an influence on the stability of the physiological microflora over time. Because of these results and as no negative effects in the animals were seen after ozone application, its use in shrimp RAS appears adequate.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Felix Teitge, DMV
Fish Disease Research Unit
University of Veterinary Medicine
Hannover, Germany -
Christina Peppler
Polyplan GmbH
Bremen, Germany -
Dieter Steinhagen, DMV
Fish Disease Research Unit
University of Veterinary Medicine
Hannover, Germany -
Verena Jung‐Schroers, DVM
Corresponding author
Fish Disease Research Unit
University of Veterinary Medicine
Hannover, Germany
Tagged With
Related Posts

Aquafeeds
Gamma irradiation enhances nutritional value of animal byproducts
Gamma irradiation treatment presents a possible processing technique for reducing anti-nutrients and improving the nutritive quality of many feed ingredients.

Innovation & Investment
Ozone increases productivity in RAS systems
Studies on improving water quality in recirculating aquaculture systems has identified ozone as an excellent solution for an optimal water environment.

Intelligence
Ozone: Sanitizer, disinfectant for seafood processing
Many chemicals are used as antimicrobial compounds but ozone has characteristics that make it desirable for disinfection and sanitizing.

Health & Welfare
In sea lice fight, salmon farmers phasing out hydrogen peroxide
An over-reliance on medical and chemical controls, along with warming waters, led to a surge in sea lice. With such treatments waning in effectiveness, operators turn to other, safer measures.


