Proper microbial management required after disinfection
Early mortality syndrome (EMS), also known as acute hepatopancreatic necrosis, typically affects shrimp postlarvae within 20 to 30 days after stocking and can cause up to 100 percent mortality. The Global Aquaculture Alliance estimated that annual losses to the Asian shrimp culture sector amount to more than $1 billion. The causative agent of EMS has been reported to be a bacterium, more specifically a pathogenic Vibrio parahaemolyticus strain. This bacterial species is a normal member of the natural microbiota in marine environments.
At this moment, research has been mainly oriented toward studying the pathology and etiology of EMS, although efforts to develop strategies to prevent or remedy the disease are equally – if not even more – needed. Based on the ecology of the causative agent, it seems that approaches with a focus on controlling the presence or activity of vibrios in general have a high chance of decreasing the risk of EMS outbreaks.
Pond disinfection
Disinfection of ponds – whether or not combined with pond drying – eliminates most micro- and macroorganisms, but is ineffective in achieving total microbial eradication, especially in biofilms and pond sediment. After refilling ponds, surviving organisms can benefit from the high availability of nutrients in the pond sediment and pond water, and the low abundance of other microorganisms to compete with them for these nutrients. These conditions favor the growth of fast-growing bacteria.
Considering the fact that many pathogenic vibrios, including the EMS pathogen Vibrio parahaemolyticus, are fast-growing, opportunistic bacteria able to multiply outside their hosts, pond disinfection is likely to result in their increased abundance in ponds. The introduction of shrimp postlarvae and feed in disinfected ponds adds to this effect by increasing the nutrient availability that promotes this microbial bloom (Fig. 1).
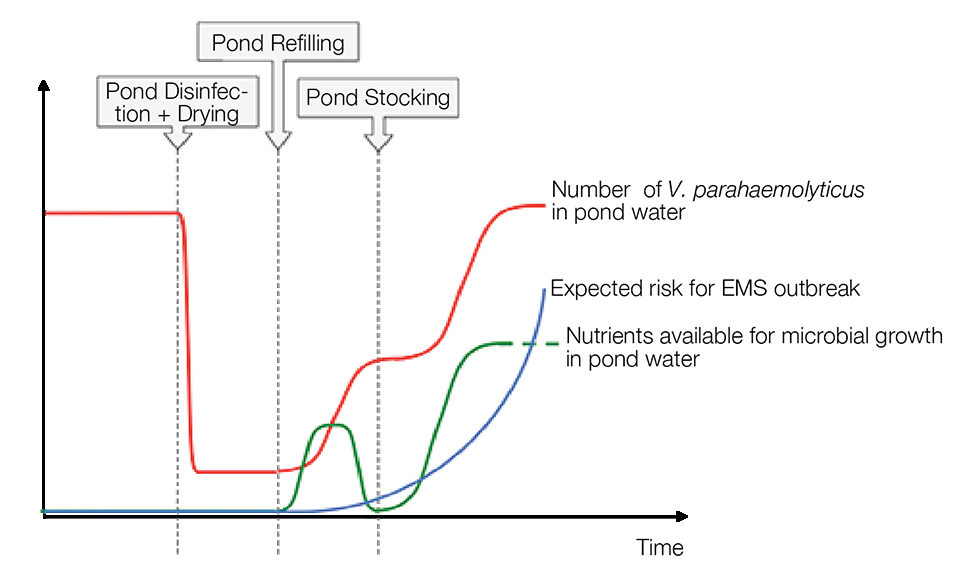
The risk of a disease outbreak is likely to increase with rising levels of the causative agent in the rearing water. Therefore, without additional follow-up management, pond disinfection can, in the long run, increase rather than decrease the risk for EMS.
In fact, EMS outbreaks resemble the outbreaks of luminescent vibriosis in the 1990s. This disease is caused by bacteria belonging to the Harveyi clade of vibrios, of which V. parahaemolyticus is also a member. Like EMS, luminescent vibriosis occurred during a typical 10- to 45-day time frame following stocking of shrimp postlarvae in grow-out ponds. Outbreaks of the disease were in general preceded by a substantial increase in the number of vibrios in the pond water following pond disinfection.
Mature microbial community
High numbers of V. parahaemolyticus in rearing water can be avoided by colonization of the water with a mature and diverse microbial community prior to stocking. This creates an equilibrium between the density of microorganisms and the level of available nutrients in the rearing water, which in the authors’ opinion is the best mechanism to prevent the EMS-causing vibrios from reaching high densities and causing a disease outbreak.
The key to establishing a mature and diverse microbial community in culture ponds is allowing a conditioning period after disinfection but prior to stocking, during which nutrients are added to promote microbial growth. This initially results in a high abundance of fast-growing bacteria that will subsequently gradually be replaced by a large diversity of slower-growing microorganisms in a mature community. Nutrients can be added, for example, by culturing tilapia in ponds during the conditioning period.
Mature water approach
The potential of the mature water principle was recently illustrated during culture of Atlantic cod larvae by Dr. Kari Attramadal and Prof. Olav Vadstein at the Norwegian University of Science and Technology in Trondheim, Norway. They compared the application of a flow-through system – which selects for fast-growing microorganisms in the culture water – with two mature water systems, one in flow-through mode and one in recirculation mode. It was observed that the microbial community in the mature water was much more diverse and stable, and that the survival of the larvae reared in the mature water systems was 72% higher than in the non-mature system.
There are indications from practice that the mature water approach will also work in shrimp culture. For example, it has been observed that EMS is less prevalent in ponds colonized by copepods. This indicates naturally mature ecosystems, as copepods require constant amounts of phytoplankton and bacteria as feed.
Greenwater – often induced by tilapia co-culture – and biofloc technology systems have also been associated with lowered incidence of EMS in practice. Such systems are characterized by mature microalgal and bacterial communities, and have been shown to result in decreased vibrio levels and decreased animal mortality. The bacteria present in these systems are able to effectively compete with the EMS-causing pathogens for available nutrients and as such to control their presence.
It needs to be stressed that the mature ecosystem approach aims at preventing EMS and does not cure EMS-infected shrimp. Therefore, farms should make sure the larvae used for stocking are free of EMS.
Perspectives
The recent outbreaks of early mortality syndrome suggest that modern intensive shrimp-farming practices need to be critically reviewed. The authors argue that the use of only disinfectants and antibiotics will not solve the problem. One should rather take advantage of the natural competition among microorganisms to keep EMS-causing bacteria from reaching high densities in culture systems.
Editor’s Note: This article was summarized from “Early Mortality Syndrome Outbreaks: A Microbial Management Issue in Shrimp Farming,” published April 24 by Dr. Peter De Schryver and co-authors in PLOS Pathogens, a journal of the Public Library of Science.
(Editor’s Note: This article was originally published in the July/August 2014 print edition of the Global Aquaculture Advocate.)
Authors
-
Dr. Peter De Schryver
Laboratory of Aquaculture and Artemia Reference Center
Department of Animal Production
Ghent University
Rozier 44
9000 Ghent, Belgium -
Dr. Tom Defoirdt
Laboratory of Aquaculture and Artemia Reference Center
Department of Animal Production
Ghent University -
Dr. Patrick Sorgeloos
Laboratory of Aquaculture and Artemia Reference Center
Department of Animal Production
Ghent University
Tagged With
Related Posts

Intelligence
An inside look at Sino Agro Foods’ giant prawn MegaFarm
Sino Agro Foods has developed a proprietary recirculating aquaculture system that yields high production volumes and profitability. The facility should significantly contribute to seafood production in China and to help satisfy increasing demand for high value, safe and sustainably produced seafood.

Aquafeeds
A look at protease enzymes in crustacean nutrition
Food digestion involves digestive enzymes to break down polymeric macromolecules and facilitate nutrient absorption. Enzyme supplementation in aquafeeds is a major alternative to improve feed quality and nutrient digestibility, gut health, compensate digestive enzymes when needed, and may also improve immune responses.

Health & Welfare
Asepsis key to prevent contamination in shrimp hatcheries
Maintaining biosecurity and asepsis in larval shrimp production is a key component of the production chain in Ecuador, which requires the production of 5.5 billion larvae monthly from 300-plus hatcheries.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.


