Fish life stage more important modulating intestinal microbiota than diet

The olive flounder (Paralichthys olivaceus) is the most economically important carnivorous marine fish cultured in the Republic of Korea, and accounts for more than 45 percent of all farmed fish production.
Fishmeal remains the gold‐standard protein ingredient for carnivorous aquaculture fish. In olive flounder, a high proportion of fishmeal is generally used to support the growth and health in the juvenile stage, and then it is progressively reduced during the grow‐out stage (~30 to 40 weeks). To reduce the reliance on fishmeal and support the sustainable future development of Korean aquaculture, researchers and producers have worked on cost‐effective alternatives to fishmeal.
Many plant proteins have been explored as fishmeal alternatives in aquafeeds, but the presence of some antinutritional factors or nutritional imbalances may negatively affect fish growth, gut microbiota composition, immune response and survival rates.
Gut microbiota are critical to the host’s nutrition, development, immunity, and resistance against stressful conditions. The advent of next‐generation sequencing, NGS [several modern sequencing technologies that allow for sequencing of DNA and RNA much more quickly and cheaply than previously used technologies, and which have transformed the study of molecular biology] enabled more sophisticated analysis of complex gut microbiota by a culture‐independent approach with unprecedented resolution and throughput.
NGS has been used to explore the dietary effects on gut microbiota of different fish species. Most of these studies investigated the dietary effects on fish gut microbiota for a short‐term, but the long‐term dietary effects at different growth stages have generally been overlooked. The dietary effect of plant protein ingredients on gut microbiota of the olive flounder has not been investigated.
This article – adapted and summarized from the original publication [Niu, K-M. et al. 2020. Dietary effect of low fishmeal aquafeed on gut microbiota in olive flounder (Paralichthys olivaceus) at different growth stages. Microbiology Open 9(3), March 2020.] – reported on an investigation of the gut microbiota of olive flounder at different growth stages with long‐term dietary administration of plant‐based low fishmeal and practical fishmeal diets by a culture‐independent metagenomic [permitting comparisons of genetic material from multiple samples] approach.
This study was supported by a grant from the National Institute of Fisheries Science (R2019012), Republic of Korea.
Editor’s note: This article has 10 co-authors (see article tags below), but only the affiliation and contact information for one of the corresponding authors – Dr. Soo Ki Kim – is listed.

Study setup
We formulated and tested two isonitrogenous and isolipidic diets as a fishmeal (FM)-based control diet (Con) and a plant protein-based low-FM diet (FM30) with 30 percent fishmeal replacement using soybean meal, corn gluten meal and corn concentrate. The two diets were produced by thoroughly mixing the feed ingredients, followed by extrusion. The pellets were then air-dried at 60 degrees-C for three hours and stored at minus-20 degrees-C until use.
The feeding trial was conducted at Aquafeed Research Center (Pohang), National Institute of Fisheries Science (NIFS), Republic of Korea. Juvenile olive flounder (average initial body weight, 30 grams) were obtained from the Korea NIFS and acclimated to environmental conditions for eight weeks while fed with the Con diet prior to the experiment.
The fish were reared in an indoor flow-through system with standard conditions, and the water temperature ranged from 16.8 to 26.1 degrees-C. After acclimation, 300 fish in each treatment fed with the Con or FM30 diet were randomly distributed into three polyvinyl circular tanks (100 fish/tank; volume, 400 liters) supplied with seawater at a flow rate of 20 liters per minute and aeration.
The feeding trial was conducted for eight months, and all the fish were fed twice a day ad libitum. Three fish, under 100 grams, were collected before the initiation of the experiment and designated the juvenile stage (<100 grams). Fish sampled during the study were designated as the grower (~400 grams), subadult (~800 grams) and adult (>1,000 grams) growth stages based on their body weight and length (Fig. 1).

For detailed information on experimental design and diets, fish and study conditions; sample collection, DNA extraction and sequencing; sequencing data analysis; and statistical analyses, refer to the original publication.
Results and discussion
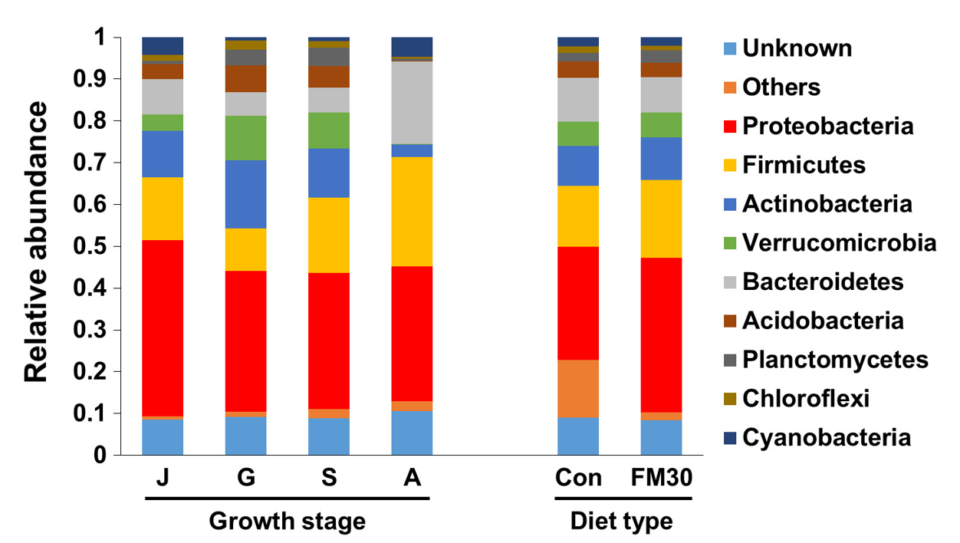
Fish were fed one of the two experimental diets for eight months, and we collected the midgut contents to analyze the gut bacterial community. We determined that there were nine dominant phyla, which in turn presented Proteobacteria, Firmicutes, and Actinobacteria as the three major phyla in the gut microbiota of the flounder. At genus level, the dominant genera were Delftia, Prevotella and Chthoniobacter at the juvenile stage (below 100 grams per fish); Chthoniobacter, Bacillus and Bradyrhizobium at the grower stage (400 grams per fish); Chthoniobacter, Bacillus and Delftia at the subadult stage (800 grams per fish); and Lactobacillus and Prevotella at the adult stage (over 1,000 grams per fish).
The microbial diversity in olive flounders increased from the juvenile and subadult stages and reached a plateau thereafter. The fish fed the FM30 diet had a significantly increased abundance of Lactobacillus and Photobacterium and had less abundance of Prevotella and Paraprevotella than the control. However, the effect of dietary plant‐based protein sources (PPS) was not significant on total microbial richness, indicating no negative effect as feed sources on the intestinal microbiota in olive flounder.
Based on our findings, the gut bacterial composition was not significantly influenced by diet until the adult stage, whereas obvious shifts of the gut bacterial community were observed at different growth stage. The microbiota of the grower fish was characterized by the highest diversity measurements, such as number of observed species, in contrast to juvenile, subadult and adult fish. Generally, a high diversity is regarded beneficial for host health.
Our results indicate that the life stage of olive flounder is more important in modulating intestinal microbiota than is the diet. It could also be concluded that dietary PPS might be used as a potential fishmeal alternative without any compromising effects on microbial diversity of olive flounder for long‐term feeding.
Gut microbiota plays important roles in nutritional, functional and physiological activities of the host. Several factors including intrinsic (i.e., age) and extrinsic (i.e., diet) may affect the fish gut microbial diversity, function and metabolic activities. To date, little information regarding the change in gut microbiota at different growth stages of olive flounder had been reported. Therefore, understanding its composition in response to diet change over its lifetime will be
ery valuable for establishing practical, low-fishmeal aquafeeds for olive flounder.
We observed that the bacterial communities were distinctly grouped according to their growth stages but were in close relationship among the individuals of different dietary groups. Overall, in comparison with the dietary effect, there was clear modulation of the growth stage on the gut microbiota in olive flounder. Other studies also reported no significant effects of the dietary treatments on the gut microbiota in rainbow trout and shrimp, respectively; however, they observed significant differences in the gut bacterial community at different growth stages.
In our study, Firmicutes and Proteobacteria were identified as biomarkers in different developmental stages in relation to low-fishmeal diets, suggesting that these phyla were prevalent in the gut microbiota of olive flounder and different species of these phyla may perform different functions in the gut ecosystem. However, further studies are warranted on the functions of these gut microbes to understand their roles in the gut of olive flounder.
Perspectives
Our study described the gut microbial profile of farmed olive flounder with long‐term use of a low-fishmeal diet and using an NGS method for the first time. We found a close relationship between the gut microbial composition and growth stage of olive flounder. The FM30 diet had subtle effects on altering the gut microbiota in the early growth stage of olive flounder. However, the abundance of Lactobacillus and Photobacterium was significantly increased after the FM30 administration for eight months.
These results could possibly provide valuable information to establish a successful low-fishmeal or fishmeal-free aquafeed for the host. Further studies need to delineate the specific changes in the overall health of the host, including growth performance, immune response, mortality, physiological parameters and functional genomics in response to the low-fishmeal diet across different growth stages.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Author
-
Dr. Soo-Ki Kim
Department of Animal Science and Technology
Konkuk University, Seoul 05029, Korea[114,107,46,99,97,46,107,117,107,110,111,107,64,109,105,107,105,107,111,111,115]
Tagged With
Related Posts

Health & Welfare
Olive flounder culture in South Korea
The Republic of Korea is by far the top global producer of olive flounder raised mainly in flow-through systems at land-based facilities on the coast.

Health & Welfare
Korean researchers study protein levels, P:E ratios in olive flounder diets
The authors conducted research to determine the optimum dietary protein levels and protein:energy ratios for different age groups of olive flounders.

Health & Welfare
Characterization of bacterioplankton communities in a Senegalese sole RAS hatchery
Study investigates composition of bacterioplankton communities in an RAS hatchery used for the production of Senegalese sole juveniles.

Health & Welfare
Dietary organic acids improve gut health, disease resistance in olive flounder
A study evaluated the effects of two organic acid blends on performance, gut health and disease resistance in olive flounder. The dietary organic acids were effective in lowering total gut bacterial counts, gut Vibrio counts and in conferring resistance against Edwardsiella tarda.


