Addressing disease-causing bacterial pathogens in Korea

Olive flounder (Paralichthys olivaceus) is a commercially important species of flatfish that is farmed in Korea, China and Japan. It is estimated that approximately 50,000 metric tons (MT) per year of this species are raised in Korea, making the country the most important producer.
The fish are cultured commercially at very high densities, and common disease outbreaks are regarded as a major hurdle constraining industry expansion. Vibrio, Edwardsiella and Streptococcus species are some of the major disease-causing bacterial pathogens in the Korean flounder industry.
Antibiotics
The routine use of antibiotics at flounder farms has been implicated in increasing the incidence of antibiotic resistance in bacterial pathogens afflicting fish, potentially causing risks for farmed fish as well as humans and other animals. Concerns have also been raised relative to the potential accumulation of antibiotic residues in the environment and in the flesh of fish, which could pose a threat to human consumers. Moreover, antibiotics have been used as growth promoters, and alternatives are a major area of interest.
As the use of antibiotics without prescription has been banned in Korea since July 2012, there is growing consensus that the future sustainability of the industry will depend upon application of alternative preventive strategies.
Organic acids
Organic acids have been considered as an alternative to antibiotics based on their ability to improve production performance and to help control bacteria and fungi in feed and in the guts of fish. Organic acids are short-chain (C1-C7) volatile fatty acids or weak carboxylic acids.
Organic acids such as formic, propionic, lactic, sorbic, benzoic, fumaric and butyric acids have typically been used in feed and food preservation. There is a great deal of interest in using organic acids to control disease and enhance performance in aquatic animals. Blends of organic acids have been successfully tested and are currently being used to control several species of pathogenic bacteria in fish and shrimp.
The authors conducted research to evaluate the effects of two different organic acid blends on the performance, gut microbial bacterial composition and resistance to Edwardsiella tarda infection in olive flounders. E. tarda is a common pathogen that causes significant losses in the culture of marine fish.

Growth trial
A 10-week growth trial was conducted in an indoor semi-recirculating aquaculture system at the Feeds and Foods Nutrition Research Center of Pukyong National University in Busan, Korea. A basal diet formulated to contain approximately 52 percent crude protein consisted mainly of fishmeal, squid liver powder, dehulled soybean meal and corn starch.
The basal diet served as the control diet. Three other diets similar in all respects were formulated, except they contained either the antibiotic oxytetracyline (OTC) at 50 mg/kg body weight/day or one of two proprietary combinations of concentrated granular organic acid products – O.A. Blend 1 (Mera™Cid, based on formic and propionic acids) at 4g/kg of feed or O.A. Blend 2 (ActivateDA™, based on benzoic and fumaric acids) at 4 g/kg of feed.
Five replicate groups of juvenile flounders with initial weights of 3.5 grams were fed the control and treatment diets at a rate of 3 percent body weight daily for the trial period. The water temperature was maintained at 20 degrees-C, and all other water quality parameters were maintained as per the requirements for this species.
At the end of the trial, fish were anesthetized and weighed for estimation of performance. Intestinal samples taken from each of the groups of fish were pooled, homogenized and plated on agar for total gut bacteria or gut Vibrio counts.
Challenge trial
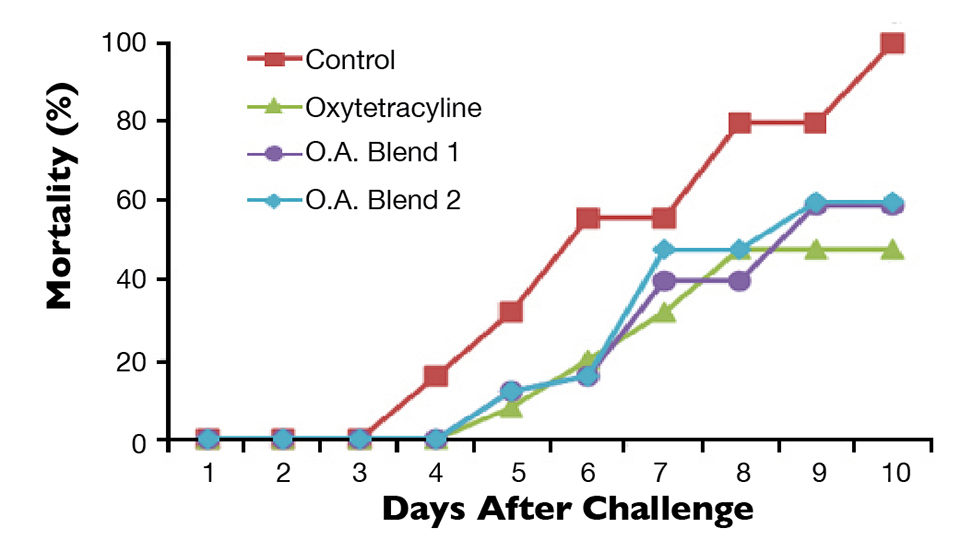
At the end of the growth trial, a challenge trial was conducted. Five fish from each replicate group in the growth trial were injected intraperitoneally with Edwardsiella tarda at 1 x 106 CFU/mL. The mortality of fish from each group was recorded over a 10-day period.
Results
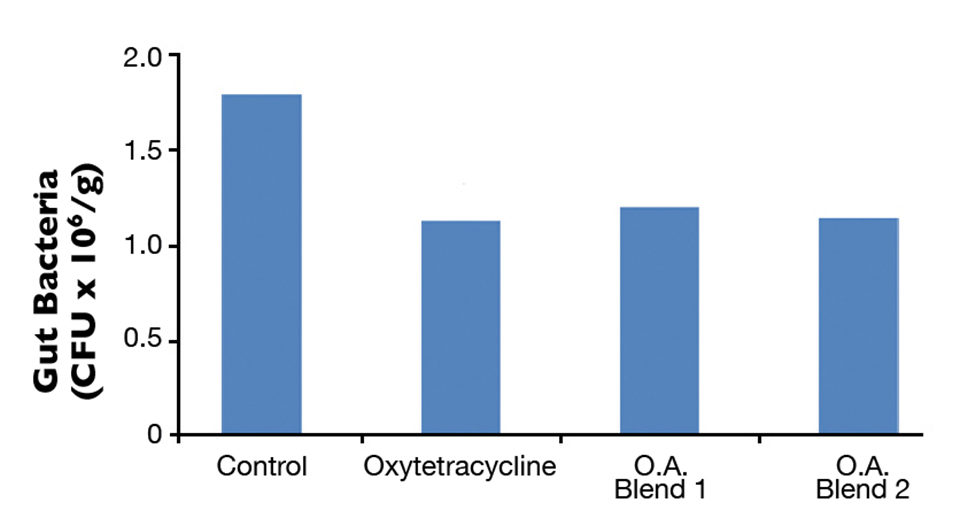
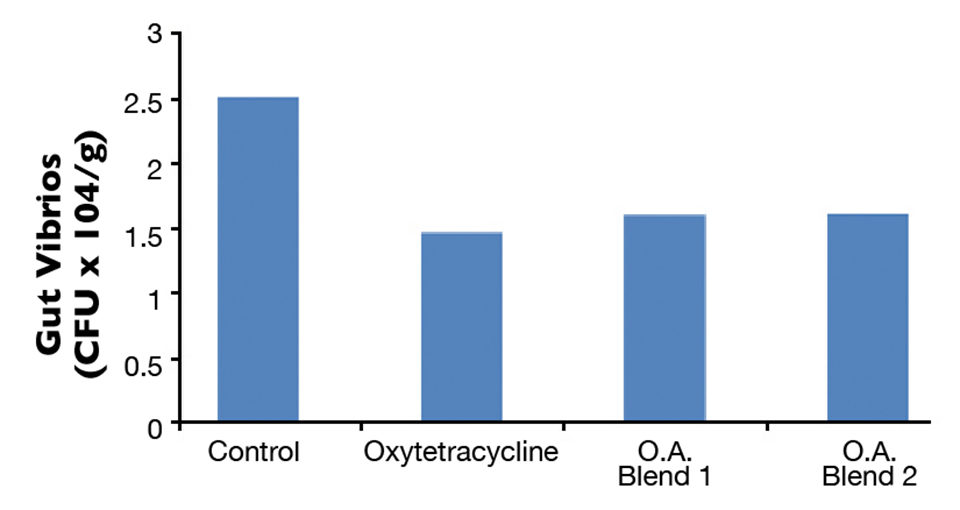
At the end of 10 weeks, total gut microflora values were significantly higher in the control group compared to the OTC and organic acid groups (Fig. 1). There was no difference in total gut bacteria counts between the OTC and organic acid treatment groups. Fish fed the diets with organic acids or oxytetracyline also had lower gut Vibrio counts compared to the control group, although not significantly different at the 0.05 level (Fig. 2). Differences in growth and survival performance were not statistically significant among the treatments. Results from the challenge study indicated 50 percent cumulative mortality after 10 days in the treatment groups a level significantly lower than the 100 percent mortality observed for the control group (Fig. 3). There was no difference in mortality between the OTC and organic acid treatment groups.
(Editor’s Note: This article was originally published in the January/February 2013 print edition of the Global Aquaculture Advocate.)
Authors
-
Gunhyun Park
Department of Marine Bio-materials & Aquaculture
Feeds and Foods Nutrition Research Center
Pukyong National University
599-1 Daeyeon 3-Dong Nam-gu
Busan 608-737 Korea -
Sungchul C. Bai, Ph.D.
Department of Marine Bio-materials & Aquaculture
Feeds and Foods Nutrition Research Center
Pukyong National University
599-1 Daeyeon 3-Dong Nam-gu
Busan 608-737 Korea -
Anant S. Bharadwaj, Ph.D.
Novus International, Inc.
St. Charles, Missouri, USA -
Craig L. Browdy, Ph.D.
Novus International, Inc.
St. Charles, Missouri, USA
Tagged With
Related Posts

Health & Welfare
Korean researchers study protein levels, P:E ratios in olive flounder diets
The authors conducted research to determine the optimum dietary protein levels and protein:energy ratios for different age groups of olive flounders.

Aquafeeds
Natural feed additives enhance rainbow trout performance
Various additives, many of plant origin, have been explored to replace the use of antibiotics in aquafeed. The authors compared the performance of rainbow trout fed diets containing oxytetracycline or mineral additives yellow loess and Song-Gang stone powder.

Aquafeeds
Salmon testes meal potential ingredient for Pacific threadfin diets
A study evaluated the potential of pink salmon testes meal, a fisheries byproduct, as a feed ingredient to replace fishmeal in diets fed to juvenile Pacific threadfin. Results showed the salmon testes meal contained high levels of protein, taurine and arginine.

Health & Welfare
Dietary organic acids used as growth promoters, anti-microbials
A feeding trial measured growth, nutrient utilization and faecal/gut bacterial counts in triplicate groups of red hybrid tilapia (Oreochromis sp.). Study results show that dietary organic acids can potentially replace OTC as a growth promoter and anti-microbial in tilapia feeds.


