Lab and field trials show positive results, cost-effectiveness

The most widely farmed shrimp species, the Pacific white shrimp (Litopenaeus vannamei), has found its global niche largely as a result of the availability of Specific Pathogen Free (SPF) animals. SPF animals are not free of all pathogens nor are they resistant or even necessarily tolerant to the pathogens that they are free of. They are a result of specific culture practices, and unfortunately, the term is widely misused. Nonetheless, they are valuable tools in many production environments and an element in lessening the overall impact of disease. They are, however, only one tool of many that can help shrimp farmers.
Despite the fact that many consider invertebrates to be phylogenetically primitive, they are far from it. The penaeid shrimp have a sophisticated immune response. It is non-specific in nature, although some of its aspects suggest that there may be some specificity. Shrimp have no memory of prior exposure to pathogens and do not form antibodies. Vertebrates, on the other hand, produce white blood cells that remember the exposure so that they are able to react much more quickly should they be exposed to the pathogen again. Shrimp do not have this mechanism. As with almost all living organisms, shrimp also have the ability to produce heat shock proteins (chaperone molecules) in response to stress. These are also involved in how the shrimp deals with the presence of pathogens.
Utilizing the shrimp immune system
Early data showed that it was possible to exploit penaeid immune systems (Lewis, D. & A. Lawrence. Immunoprophylaxis to Vibrio sp. in pond reared shrimp. Proceedings of the first International Conference on Warm water Aquaculture-Crustacea. p. 304-307. 1983). However, we now know that their mechanisms of protection are not just related to what would be characterized as being solely due to the presence of different classes of lymphocytes.
Shrimp are quite elegant in how they deal with pathogens. Exploitation of their immune response has not been met with a great deal of success in the field. The reasons for this are complex but center around the inherent nature of the processes by which shrimp are produced, socio-economic conditions, large gaps in biosecurity, corruption and greed.
Aquaintech Inc. has developed and field tested a parabiotic (two naturally or experimentally united organisms) that clearly benefited shrimp in lab trials and large-scale field trials. The data clearly show a cost benefit, and most tests were significant (p < 0.05). Extensive laboratory trials were conducted with the parabiotic before it was field tested, because the manner in which shrimp are tested can be problematic. In our experience, many products that appear to offer benefits in the lab fail to do so in the field; in fact, most do so.
One of the reasons for these failures likely relates to how shrimp eat. Many products are tested in the lab by being added directly or indirectly to feed. Shrimp grind their feed with their mouth parts before they ingest it. After it enters the stomach, it is ground further by the gastric mill before it enters the hepatopancreas and the intestinal tract. Most of what is consumed is going to be present as particulate material that the shrimp are likely ingesting through other modes (gills, with water intake, etc.). Essentially, during aquarium studies the shrimp are covered repeatedly in the material, and this does not occur in the field.
The parabiotic is added to postlarvae (PL) tanks, and the PLs are held at high densities for the duration of the feeding test. The parabiotic is fed at range of dilutions, from 1:500 to 1:5000, depending on the particular approach being used. Typically, water levels in tanks are lowered to facilitate uptake of the concentrated material. High levels of aeration are required to minimize stress and the PLs are held for a minimum of three hours. They are then removed, and the process is repeated with naive animals as needed.
Lab test results: Survivals post challenge
Laboratory trials were conducted by many different commercial groups and the results demonstrated that animals were able to tolerate exposure to both viral and bacterial pathogens. It should be noted that this is not black and white, much as field results generally are. We still do not have a discretely identified mechanism yet that explains the range of the observed results, but are working on this.
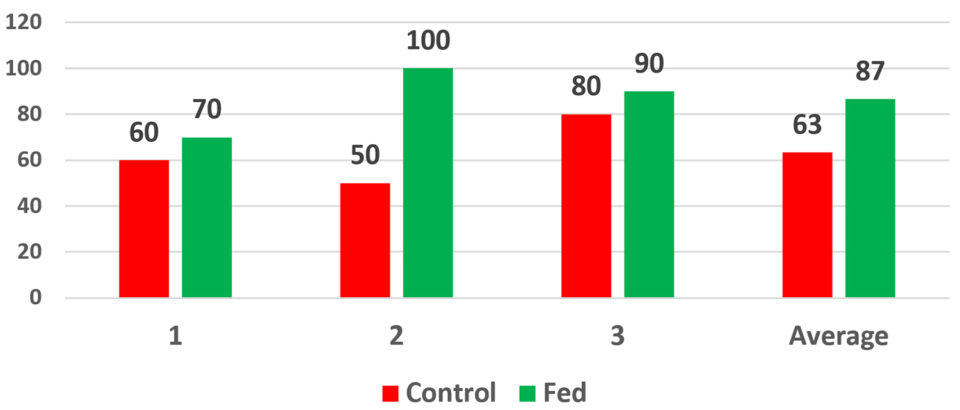
Fig. 1 presents the results of PL15 – in experimental batches of 30 PLs – fed the parabiotic at the Mysis 3 larval stage while challenged with 105 CFU/ml (colony forming units per ml) of Vibrio parahaemolyticus. Tests with a control (natural) challenge showed similar impacts. Overall, the test results showed that, under the conditions of the challenge, the animals fed the parabiotic were less susceptible to this strain of Vibrio. The control groups experienced 60, 50 and 80 percent survivals with an average of 63 percent. The parabiotic-fed animals experienced 70, 100 and 90 percent survivals, respectively, with an average of 87 percent.
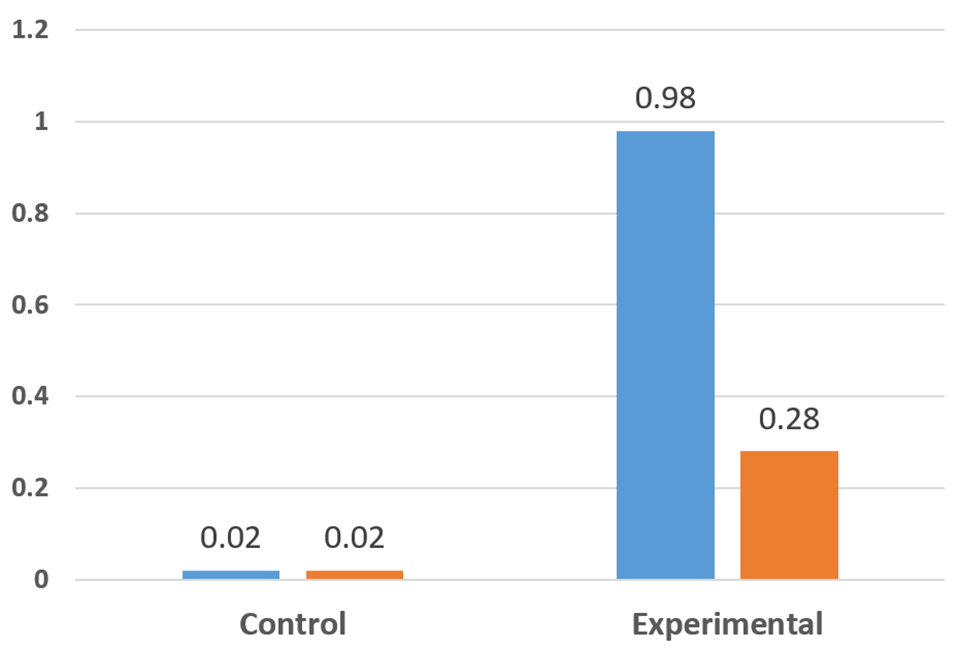
Another series of experiments involved exposing parabiotic-fed animals to tissues containing high levels of the Taura Syndrome Virus (TSV) (Fig. 2). The results clearly demonstrated that shrimp fed the parabiotic were better able to tolerate exposure to TSV. In replicate studies, 98 percent of the control shrimp died, whereas in the parabiotic fed groups, one group had a 98 percent survival and the other group 28 percent. The differences in the results reflect differences in the viral loads in the infective tissues fed to the animals. Other tests confirmed that the animals fed the parabiotic required much higher levels of exposure to the TSV to produce the same level of mortality as controls. A similar observation was noted with exposure tests to the White Spot Syndrome Virus (WSSV).
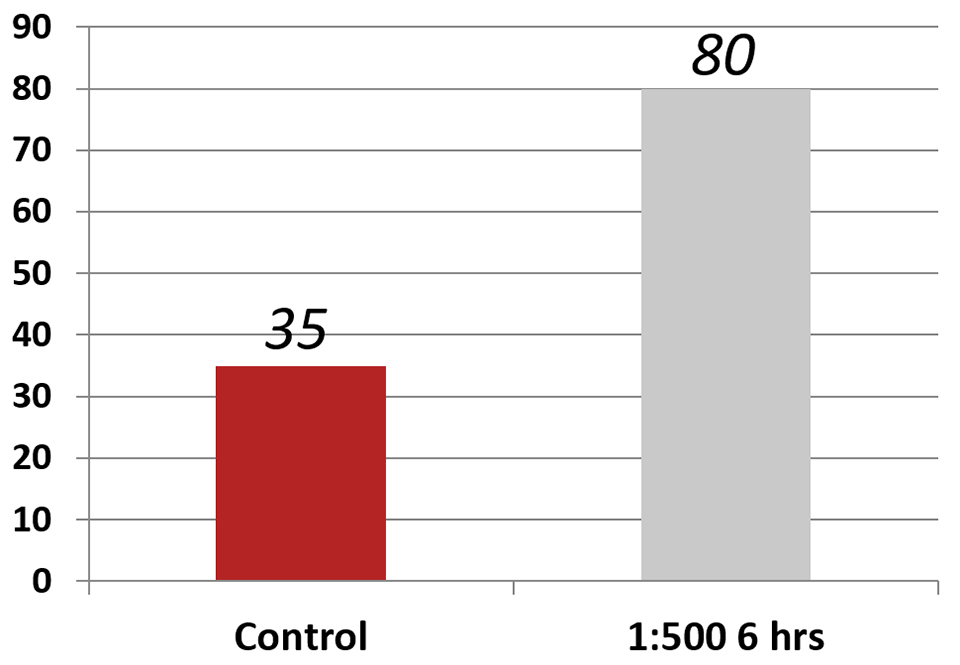
Another test involved exposing parabiotic-fed PLs to a Vibrio parahaemolyticus strain that causes Early Mortality Syndrome, or EMS (or Acute Hepatopancreatic Necrosis Disease, AHPND) (Fig. 3). These results were the average of three replicates, and results showed a clear effect. PLs fed the parabiotic at a 1:500 dilution for three hours were largely refractory to infection to the bacteria that causes EMS (AHPND), with 80 percent of the animals surviving, contrasted with only 35 percent of the control animals.
Cage testing in the field
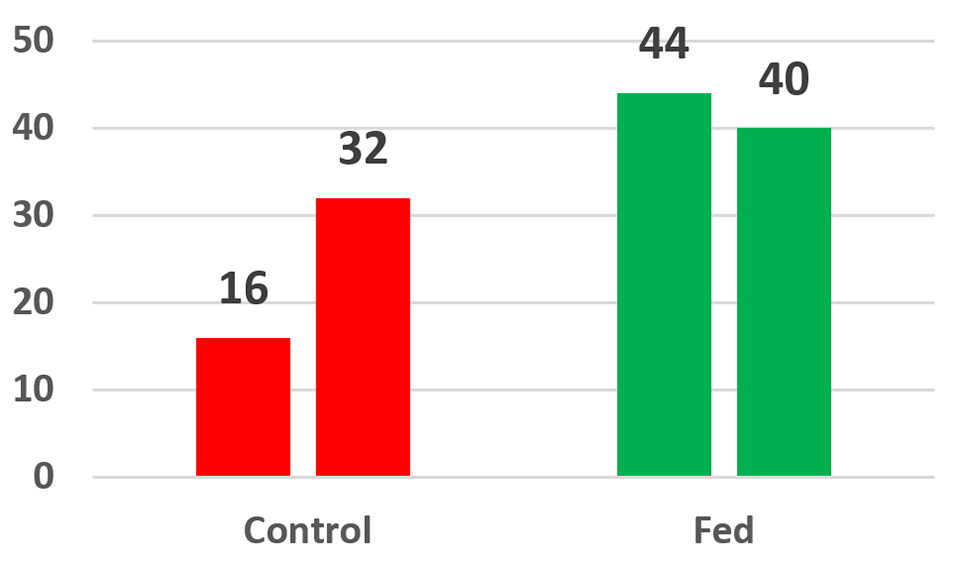
Fig. 4 shows the average survivals of two cage studies conducted at a commercial farm in Honduras. Four control cages and four experimental cages, each containing 40 animals (20 per square meter) were stocked into a single pond at two different sites on the farm (sites A and B). At 56 days the experiment at site A was terminated. Only 16 percent of the control animals were alive compared with 44 percent of the parabiotic-fed animals; this difference represented a 175 percent increase in survival. After 59 days the experiment at site B was terminated, and 32 percent of the control animals were alive compared with 40 percent of the parabiotic-fed animals; this represented a 25 percent increase in survival.
In another series of experiments (Fig. 5), a single cage was placed into each of six ponds, three controls and three experimental. At the end of the experiment, there was a significant difference in survivals with the parabiotic-fed groups consistently outperforming the controls. This set of tests demonstrated once again that shrimp fed the parabiotic prior to stocking had increased survivals.
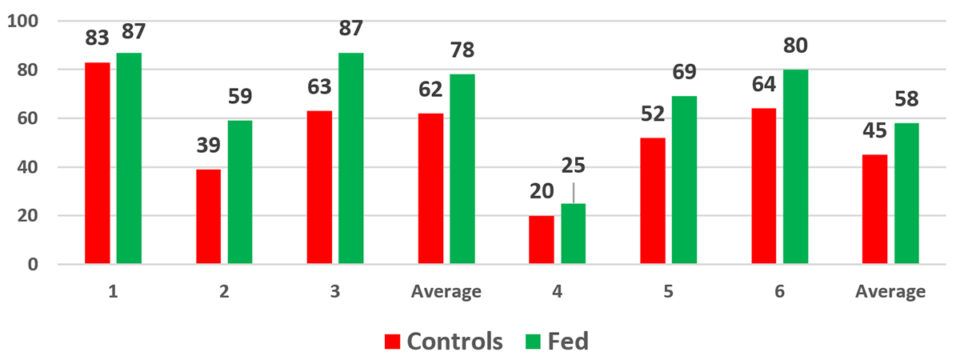
These results highlight an important observation. There must be something happening to the shrimp that the exposure to the biogenic parabiotic can impact. When survival rates in control animals are high, they are also going to be high in the parabiotic-fed animals. Conversely, if the control animals have very high levels of mortality, any beneficial impact can be overcome.
The results from extensive experiments in the field corroborate this impact. Additionally, we observed a wide range of impacts on the parabiotic-fed animals that, while clearly cost-beneficial, were not always related to any overt animal health issues. We believe that the mechanism of action involved is likely complex.
Field trials
Our parabiotic has been used on billions of PLs in the field. For the most part, there was significant cost benefits that justified the use of the product as part of a Standard Operating Procedure. Table 1 presents the results of a trial with three control and three parabiotic-fed ponds in Ecuador, at a shrimp stocking density of eight animals per square meter. Results showed significant cost-benefits, because for every $1 spent on the use of the parabiotic, the farmer realized more than a $9 increase in profit. This was calculated using a computer program that plotted a regression curve based on inputs and real-world costs, and which predicted the time at which the profit from the harvest was maximized.
Newman, parabiotic, Table 1
| Parameter | Parabiotic-fed animals | Control animals | Difference (%) |
|---|
Parameter | Parabiotic-fed animals | Control animals | Difference (%) |
|---|---|---|---|
| Survival (%) | 57.6 | 48.4 | 19 |
| Weight at harvest (g) | 9.2 | 9.6 | -5.2 |
| Yield (pounds/hectare) | 1,614 | 1,412 | 14.3 |
Results from many field trials have shown that there was a cost benefit when the parabiotic was fed late in the hatchery cycle or before stocking the ponds. It also showed a strong benefit in the hatchery (data available but not included here). Moreover, the field trial results also showed the following: 1. No two shrimp farms are the same; 2. The benefit of using the parabiotic varied; 3. The feed conversion ratios (FCRs) were improved in a number of the tests, with treated animals sometimes larger; 4. On occasions, whatever affected the animal did not seem to be influenced by consuming the parabiotic; 5. There were trials in which there was no apparent difference between the groups; this was usually a result of the presence of pathogens and other serious stressors that overwhelmed any potential benefit.
Based on all our accumulated data, we theorize that animals are impacted by the parabiotic via a short-term effect. Cage studies and early harvested field trials show a fairly consistent effect, and the lab studies demonstrate that the parabiotic-fed animals are stronger in some way. Using an analogy of racehorses where all the horses genetically the same, the first horse out of the starting gate will be the winner. Exposure to the parabiotic appears to strengthen the PLs in a manner we cannot yet explain or understand. This increase in fitness appears to give the parabiotic-fed animals an advantage under some culture conditions.
There was not always a positive benefit, although we never observe a negative effect. If the animals are in fact a bit stronger early on, then when this effect wanes it stands to reason that they then become vulnerable to whatever pathogens/stressors are present in the culture environment.
Table 2 shows our test results from a very large field trial in production ponds at a large commercial farm in Ecuador. Test A involved 24 ponds, 463 hectares, 83 million shrimp. Test B had 18 ponds, 181 hectares, 18 million shrimp harvested. Test A shrimp had low survival rates, although the parabiotic-fed animals averaged slightly better, and their average weight was also better at harvest. However, these small differences resulted in a 7.4 percent increase in the harvest yields between the groups. Even if one assumes that survivals and weights are basically the same, the 7.35 percent difference in the FCR was significant across 12 ponds, and this alone paid for the product usage many times over. Test B had similar results and demonstrated that, in this trial, the final average weight at harvest of the shrimp fed the parabiotic was almost 1 gram larger than for the control group, with a significant cost benefit.
Newman, parabiotic, Table 2
| Parameter | Parabiotic-fed animals | Control animals | Difference | Increase (%) |
|---|
Parameter | Parabiotic-fed animals | Control animals | Difference | Increase (%) |
|---|---|---|---|---|
| Test A: survival (%) | 30.7 | 29.04 | 5.8 | 1.6 |
| Test A: weight at harvest (g) | 10.6 | 10.4 | 0.2 | 1.7 |
| Test A: yield (pounds/hectare) | 1,253.7 | 1,167.3 | 86.4 | 7.4 |
| FCR | 1.89 | 2.04 | 0.15 | 7.35 |
| Test B: survival (%) | 57.3 | 56.7 | 0.6 | 1.1 |
| Test B: weight at harvest (g) | 14.24 | 13.29 | 0.95 | 7.1 |
| Test B: yield (pounds/hectare) | 1,728 | 1,609 | 119 | 7.4 |
In conclusion, our results to date indicate that our parabiotic product generally provides a significant cost benefit when used regularly, although sometimes we have seen no discernible effects. We are engaged in further research to improve our product and better understand how it works.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Author
Related Posts

Health & Welfare
Culture water pre-inoculation of probiotics in Pacific white shrimp nurseries
This study showed that a longer inoculation of probiotics will improve shrimp postlarvae growth and survival, and that a new technique developed for more accurately estimating postlarvae weight can significantly reduce the food that must be supplied.

Health & Welfare
Challenging Pacific white postlarvae with AHPND
Study results indicate that P. vannamei challenged with AHPND in biofloc had higher survival rates than shrimp challenged in clear water.

Innovation & Investment
AI platform delivers data to fish and shrimp farmers
XpertSea does more than count fish. It uses AI and computer vision to calculate growth rates and optimal harvest dates to improve aquaculture efficiency.

Health & Welfare
Antibiotics in aquaculture: Is responsible use possible?
Regulations on antibiotics in aquaculture vary by country and region, from outright bans to minimal oversight.


