Shrimp cultured with MOS-added feed had 30 percent higher survival in intensive production conditions

Regarding disease management in aquaculture, prevention is more advisable than treatment. One such strategy is to modify the intestinal microbiota [ecological communities of microorganisms found in and on all multicellular organisms] of the cultured animal to promote the colonization of beneficial bacteria and to prevent the colonization of potentially pathogenic bacteria.
Mannan oligosaccharides (MOS) are glucides [organic compounds that contain a carbohydrate] obtained from the Saccharomyces cerevisiae yeast cell. The use of MOS to block pathogen colonization is based on the knowledge that certain polysaccharides [large molecules made of many smaller simple sugars] could be used to block the mechanism of recognition and adhesion of potential pathogens to molecules on the surfaces of host tissues (competition for attachment sites). This action would reduce the adhesion of the pathogens to the digestive tract, leaving them to be excreted in the feces. This may lead to improvement of the integrity and performance of the intestinal epithelial barrier.
There are no previous publications using deep, Next Generation Sequencing (NGS, a technology to determine the sequence of DNA or RNA to study genetic variation associated with diseases or other biological phenomena) to assess the effects of MOS in the microbiota of Pacific white shrimp (Litopenaeus vannamei). Several studies have reported the effects of MOS on crustacean culture performance including parameters such as growth rates, survival, changes in gastrointestinal (GI) morphology and others. But these previous investigations were conducted under laboratory conditions and involved only cultivable bacteria. These observations have not been validated at a commercial farming scale, limiting the application of those prebiotics. And for cultured crustaceans such as L. vannamei, available information on the functionality and structuring of their microbiota is very limited.
This article – adapted and summarized from the original publication [Gainza, O., Romero, J. Effect of mannan oligosaccharides on the microbiota and productivity parameters of Litopenaeus vannamei shrimp under intensive cultivation in Ecuador. Sci Rep 10, 2719 (2020)] –reports on a study using NGS to assess the effects of the dietary inclusion of a prebiotic (MOS) on production parameters in intensive cultivation conditions of, and the composition of the intestinal microbiobiota of L. vannamei in Ecuador.
Study setup
The study was carried out at the Santa Ana intensive shrimp farm in El Oro Province, Ecuador. Approximately 1,780,000 L. vannamei juveniles (average initial weight of 2.2 ± 0.53 grams) were distributed in four, 0.5-hectare ponds under greenhouses, each with controlled water temperature (32 to 34 degrees-C), 5 ppt salinity, and dissolved oxygen saturation above 60 percent. To minimize the factors that could influence the results of the experiment, we standardized conditions such as pond maturation stage to avoid interfering with the experimental cultured shrimp.
The shrimp were cultured for 59 days until they reached harvest weight. They were fed four times per day with a commercial feed (Nicovita Classic Camarón; Vitapro, Callao, Peru) with 35 percent protein and 5 percent fat. MOS (Bio-Mos®, Alltech Inc., Nicholasville, KY, US) at 0.5 percent weight per weight (w/w) was added to the feed through the dilution of MOS in distilled water (0.125 g/mL) plus commercial gelatin (0.125 g/mL), sprayed onto the feed (0.04 mL/g) in a mechanical hopper for homogenization. The food for the two control ponds was prepared with the same protocol with the addition of commercial gelatin without MOS. Several shrimp samples were collected for different laboratory analyses.
For detailed information on the experimental design and husbandry protocols; sampling procedures; DNA extraction and PCR amplification; high-performance mass sequencing and data processing; and statistical analyses, refer to the original publication.
Results and discussion
To the best of our knowledge, this article presents the first description of the microbiota diversity and taxa composition in L. vannamei under a MOS dietary treatment in a commercial aquaculture facility. Similarly, there are no previous NGS-based reports addressing the effect of MOS on the microbiota of L. vannamei.
Previous relevant research efforts have been limited to laboratory-level experiments. In one of those studies, researchers used 1080 shrimp distributed in 36 1-cubic-meter tanks and reported a 66 percent increase in weight gain in the group treated with 0.2 percent MOS. In another study with 270 shrimp in 18, 0.128-cubic-meter aquaria with water salinity of 38 ppt, researchers reported a 17 percent increase in survival after increasing MOS to 0.4 percent.
Our results under intensive commercial cultivation conditions – with 1,780,000 juvenile L. vannamei in four 0.5-ha ponds, 5 ppt salinity and 0.5 percent MOS – contrast with previous laboratory studies because we found no significant differences in growth parameters between the animals supplemented with MOS and the control treatment. However, the increase in harvested biomass in ponds that received the 0.5 percent MOS treatment resulted from a significant 30 percent increase in shrimp survival.
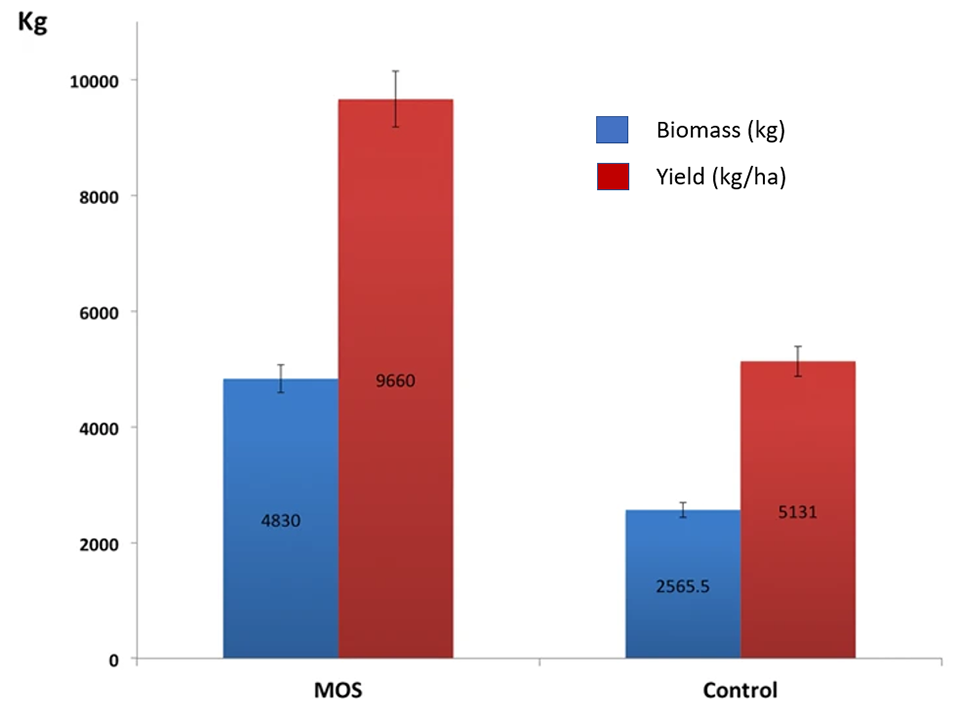
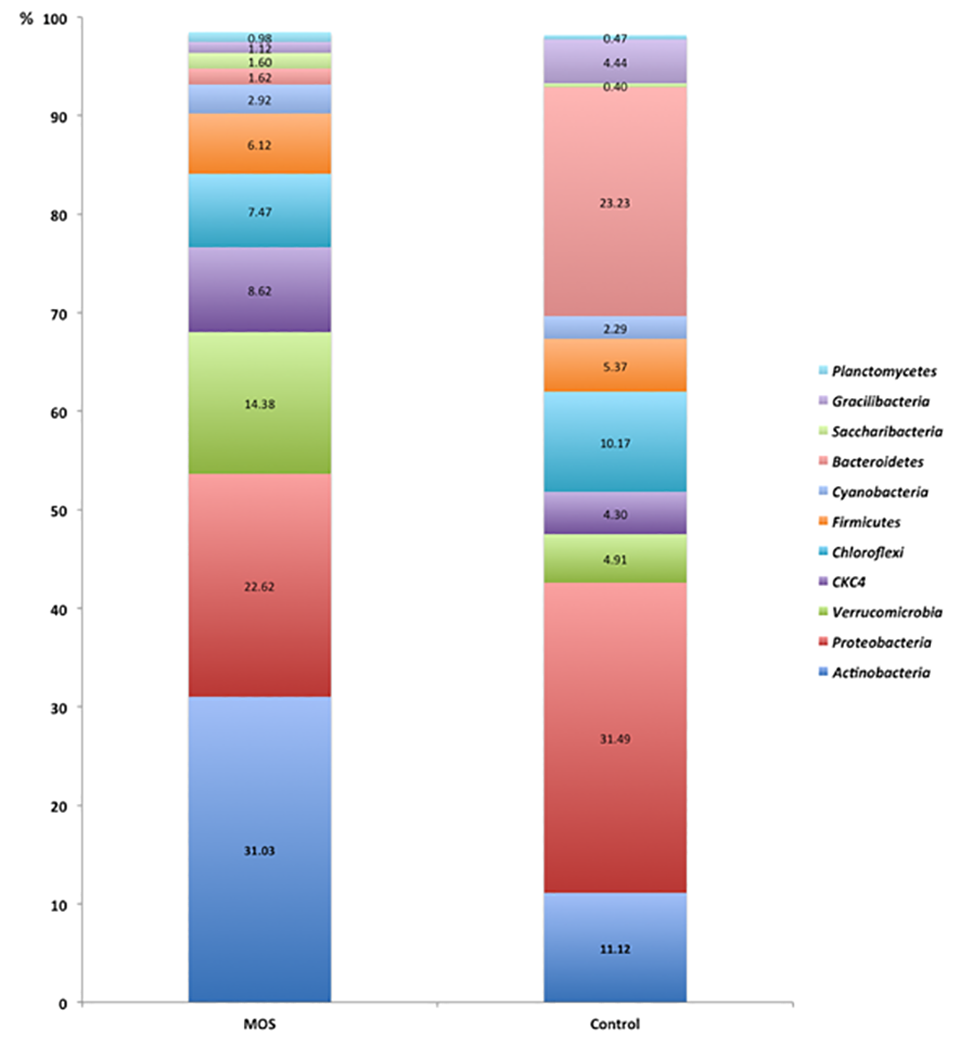
The promotion of changes in the composition of the intestinal microbiota is a key component of the case for using MOS as prebiotics, but this needs further research. The composition of the microbiota of shrimp under our controlled conditions is consistent with previous research with L. vannamei, with Proteobacteria as the dominant phylum in our study and with relative abundance levels between 28 and 70 percent. Other phyla showing high abundance under control conditions were Bacteroidetes (22 percent) and Actinobacteria (11 percent).
Considering the impact of Acute Hepatopancreatic Necrosis Syndrome (AHPNS), a serious disease of farmed shrimp, on the production of L. vannamei worldwide, our results showing the reduction of the genus Vibrio to negligible levels in a commercial culture facility are of particular relevance, because previous studies only involved laboratory-scale assays. Our work suggests that MOS could constitute an important tool for preventing AHPNS, which remains one of the most interesting open fields for exploration based on our results.
The change in the intestinal microbiota is reflected in the improved survival and better productivity outcomes and is consistent with the concept that Actinobacteria produce beneficial secondary metabolites for the host, such as antimicrobial factors and growth promoters. There are numerous publications on the potential use of Actinobacteria as probiotics, but they have all focused on the genera Streptomyces and Lactococcus. Some authors have suggested that the selection of probiotics should favor the specific microbiota of each species to provide a greater probability of intestinal colonization. Based on our results, potential probiotics belonging to the genera Actinomadura, Fodinicola and Agromyces should be evaluated for their abundance and association with MOS.
Overall, our results showed that the MOS inclusion increased L. vannamei shrimp survival by 30 percent in a commercial production setting. Also, the use of NGS revealed quantitative differences in the shrimp microbiota between MOS and control conditions. In the treatment with inclusion of dietary MOS, the predominant phylum was Actinobacteria (28 percent); while the control group was dominated by the phylum Proteobacteria (3 percent).
Under the MOS treatment, the prevalence of potential opportunistic pathogens in the experimental shrimp – like Vibrio, Aeromonas, Bergeyella and Shewanella – was negligible. This may be attributable to MOS blocking the adhesion of pathogens to the surfaces of the host tissues. Together, these findings indicate that the performance (survival) improvements of the dietary MOS may be linked to the impact on the microbiota, since bacterial lines with pathogenic potential towards shrimps were excluded in the gut.
Perspectives
Our study is first approach through NGS to the management of the microbiota modification of L. vannamei under commercial cultivation conditions. In 2010, Daniels et al. reported that the action of MOS stabilizes the composition of the microbiota and partly suppresses the variations and influxes of new bacterial strains from the environment. This statement is fully consistent with the results of our diversity analysis and may be extended to say that the action of the MOS also controls the influx of bacterial strains with potential pathogenicity for L. vannamei.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Oreste Gainza, Ph.D.
Departamento de Acuicultura, Universidad Católica del Norte, Doctorado en Acuicultura, Programa Cooperativo Universidad de Chile, Pontificia Universidad Católica de Valparaíso, Universidad Católica del Norte, Larrondo 1281, Coquimbo, Chile
-
Jaime Romero, Ph.D.
Corresponding author
Laboratorio de Biotecnología de Alimentos, Unidad de Alimentos, Instituto de Nutrición y Tecnología de los Alimentos (INTA), Universidad de Chile, El Líbano 5524, Macul, Santiago, Chile
Tagged With
Related Posts

Innovation & Investment
Innovation Award 2020 finalist: ME-PRO® from Prairie AquaTech
An alternative aquaculture feed ingredient made in the American Plains is a finalist for GAA’s annual Global Aquaculture Innovation Award.

Health & Welfare
Prebiotics, probiotics provide alternatives to antibiotics
As the demand for aquaculture products increases, so does the search for environmentally friendly alternatives to antibiotics. Alternatives to antibiotics include dietary prebiotics, probiotics and synbiotics.

Health & Welfare
Aiding gut health with a natural growth promotor
A study with Nile tilapia conducted in commercial production cages in Brazil showed the potential – in the absence of major disease threats – of a commercial, natural growth promotor that modulates the microbiota (inhibiting growth of pathogenic bacteria and promoting growth of beneficial bacteria) and inhibits quorum sensing.

Health & Welfare
Intensive nursery, pre-grow-out of white shrimp in autotrophic, heterotrophic systems
A seven-week trial evaluates autotrophic and heterotrophic systems in the nursery and pre-grow-out of Pacific white shrimp.


