Organic carbon source affects nutrient utilization efficiency in biofloc systems for L. vannamei

Biofloc technology is a resource-efficient aquaculture production system that supports better use of basic natural resources, such as fresh water and land, and aquafeeds. The addition of organic carbohydrate (CHO) to the biofloc system provides an energy source for microbial organisms to convert ammonia or nitrate into microbial biomass. This process helps to lower ammonia and nitrite levels, thereby reducing the need for water exchange. At the same time, the generated microbial biomass serves as natural food for the cultured species and increases feed use efficiency. Biofloc systems also benefit immunological responses of Pacific white shrimp (Litopenaeus vannamei) against infectious agents.
Potential CHO sources may include simple ones such as molasses, glycerol and glucose, and complex ones such as flours and starches. Different CHO sources result in different nutritional values of the biofloc. In addition, they have varying effects on the composition of the microbial community in the biofloc, the production, and the immunity of the cultured fish and shrimp. The underlying mechanism for some of these differences may lie in the complexity of CHO structure. These results altogether indicate the importance of selecting a suitable CHO for a successful biofloc technology system.
In biofloc technology, feed and CHO represent major sources of organic material entering the system. Following feeding, the oxygen consumption and ammonia excretion by fish significantly increase, creating high fluctuations in ammonia concentration on a daily basis. It is currently unknown how the carbon and nitrogen inputs are accumulated in different compartments (e.g. shrimp, biofloc, water) of the biofloc culture of L. vannamei, and further research on nutrient recycling in biofloc systems is still needed.
This article – adapted and summarized from the original publication [Tinh, T.H. et al. 2021. Effects of carbohydrate sources on a biofloc nursery system for whiteleg shrimp (Litopenaeus vannamei). Aquaculture, Volume 531, 30 January 2021, 735795] – investigated how the addition of corn starch or molasses affected water quality, biofloc and periphyton [complex mixture of algae, detritus, cyanobacteria and heterotrophic microbes attached to submerged surfaces in most aquatic ecosystems] proximate composition, shrimp production parameters, diurnal fluctuations and distribution of carbon and nitrogen in a L. vannamei shrimp nursery system.
Biofloc potential to alter virulence of V. parahaemolyticus strain on L. vannamei postlarvae
Study setup
The experiment was carried out at the animal research facility of Wageningen University (Carus), the Netherlands. L. vannamei (0.075 ± 0.006 grams) juveniles were obtained from CreveTec in Ternat, Belgium, and stocked at the density of 250 shrimp per square meter into tanks in an indoor nursery system. The tanks had continuous aeration, temperature control, a 12-hour/12-hour dark/light regime and established biofloc.
The animals were fed twice per day with 34 percent commercial protein feed (CreveTec) at the feeding level of 8 percent body weight (BW) per day, and an assumed feed conversion rate of FCR of 0.7, reaching a maximum feeding rate of 43 grams per cubic meter per day. After feeding, corn starch and molasses were added immediately to their respective treatments. For each kilogram of shrimp feed fed, 0.6 kg of corn starch or 1.1 kg of molasses were added to maintain an input C:N ratio of 12.
Shrimp samples were collected as a composite sample at the beginning and separately from each tank at the end of the experiment for determination of average body weight and survival rate. Biofloc samples were collected at the beginning of the experiment and the end of weeks 1, 3 and 5. Other samples were also collected at various times during the experiment.
For detailed information on the experimental design and system, and animal husbandry; sample collections and analyses; and data analyses, refer to the original publication.
Results and discussion
Overall, results of the study showed that both corn starch and molasses addition treatments resulted in low ammonium nitrogen levels in the water. The total suspended solids and volatile suspended solids in both treatments increased over time and were not significantly different among treatments. The protein content in the dry matter of the biofloc varied from 34 percent to 48 percent, being higher in the molasses treatment. The same was observed for the protein content in the dry matter of the periphyton which ranged between 16 percent and 26 percent.
The corn starch treatment resulted in significantly higher shrimp growth rate, production, average body weight and lower FCR compared to molasses addition. Water quality was stable on a daily basis, but changed over the weeks. Carbon and nitrogen accumulations in the system were not significantly different among treatments.
The survival percentage (90 to 96 percent) and specific growth rate (SGR), the increase in cell mass per unit time (8 to 10 percent body weight, BW, per day) of shrimp in this study were high and comparable to previous studies on L. vannamei shrimp nursery. We used a carbon:nitrogen (C:N) ratio of 12 for both treatments, and recommended for biofloc culture of L. vannamei shrimp.
The addition of corn starch yielded significantly better shrimp production, probably due to the more stable environmental conditions in the corn starch treatment, in particular the dissolved organic carbon and nitrogen compared to the molasses treatment (Fig. 1). Stable water quality reduces stress and improves growth and survival of cultured shrimp. Lower and more stable organic carbon and nitrogen concentrations in the water of the corn starch treatment suggest that the microbial community in the experimental system differed in composition and was more efficient than in the molasses treatment.
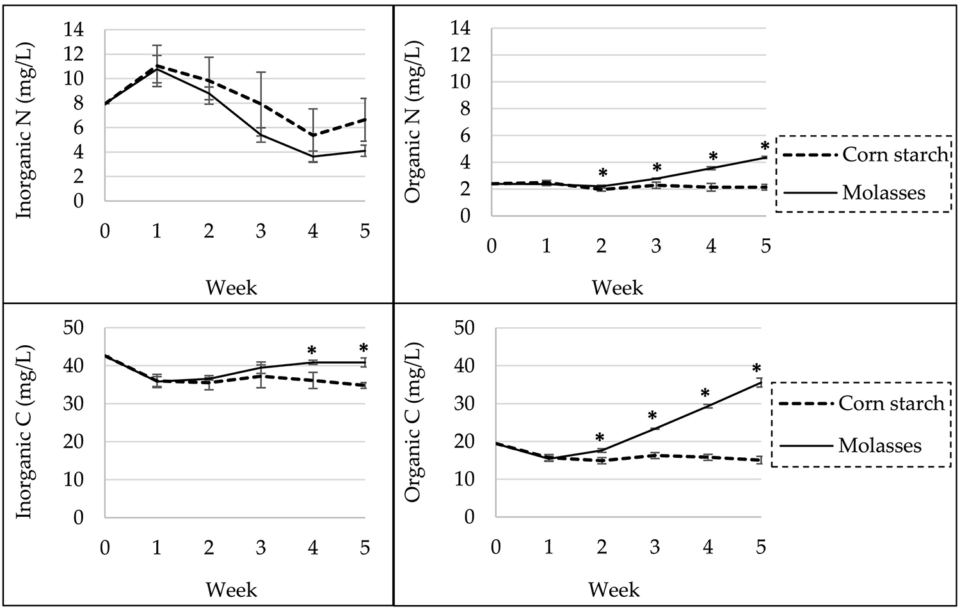
The two carbohydrate sources used in this research differed significantly in mineral content, especially in potassium. Previous research also reported that iron, potassium and manganese concentrations in molasses were approximately 17, 50 and 70 times, respectively, higher than in starches. Phosphate concentrations in the molasses treatment was also higher than in the corn starch treatment and previously reported values in conventional shrimp pond. But it is not clear if these differences contributed to the difference in shrimp growth among treatments. Overall, in addition to maintaining a suitable C:N ratio, the choice of CHO source is of major importance in biofloc technology, as different CHOs have different effects on biofloc nutritional content, microbial diversity and production of the cultured animals.
Biofloc protein content was higher than 390 g/kg dry weight in both treatments. The average biofloc concentration reached 434 mg per liter, presenting an extra source of nutrients for the cultured shrimp. The higher biofloc protein content observed in the molasses treatment likely resulted from the fact that molasses contains more protein on a dry weight basis (8.5 percent) than corn starch (0.3 percent), which was directly available to biofloc.
We observed that as the biofloc concentration increased, its protein content increased, while its ash content decreased. This may be due to changes in the biofloc microbial composition. At high biofloc concentration, the bacteria part in biofloc became dominant over the algal content. In our study, in the system was outcompeted from week 3 onwards, showing a decrease in chlorophyll a concentration when biofloc concentration reached 403 mg per liter in the corn starch and 417 mg per liter in the molasses treatments. A balanced biofloc system where neither algae nor bacteria is dominant is more beneficial for shrimp, and biofloc concentrations of 400 to 600 mg per liter are reported as suitable for L. vannamei shrimp culture.
Periphyton production in our trial reached 22 to 46 grams DW per tank at the end of the experiment, with protein contents ranging from 19 percent to 24 percent. Periphyton protein content, similarly to biofloc protein content, was significantly higher in molasses treatment. There are few studies on the effects of carbohydrate type on periphyton, but periphyton nutritional values have been shown to be dependent on substrate type. The periphyton protein level in our study was comparable to the 25 percent reported by researchers for L. vannamei ponds and represented an extra source of nutrients for the animals.
The direct contribution of periphyton to intensive culture of L. vannamei shrimp has not been studied, but scientists have shown that promoting periphyton growth by substrate addition increased L. vannamei shrimp production in less intensive systems. We observed that shrimp grazed on periphyton, which in our system mostly grew on the tank wall at the water-air interface, so that maintaining a constant culture water level may increase the availability of periphyton for the shrimp.
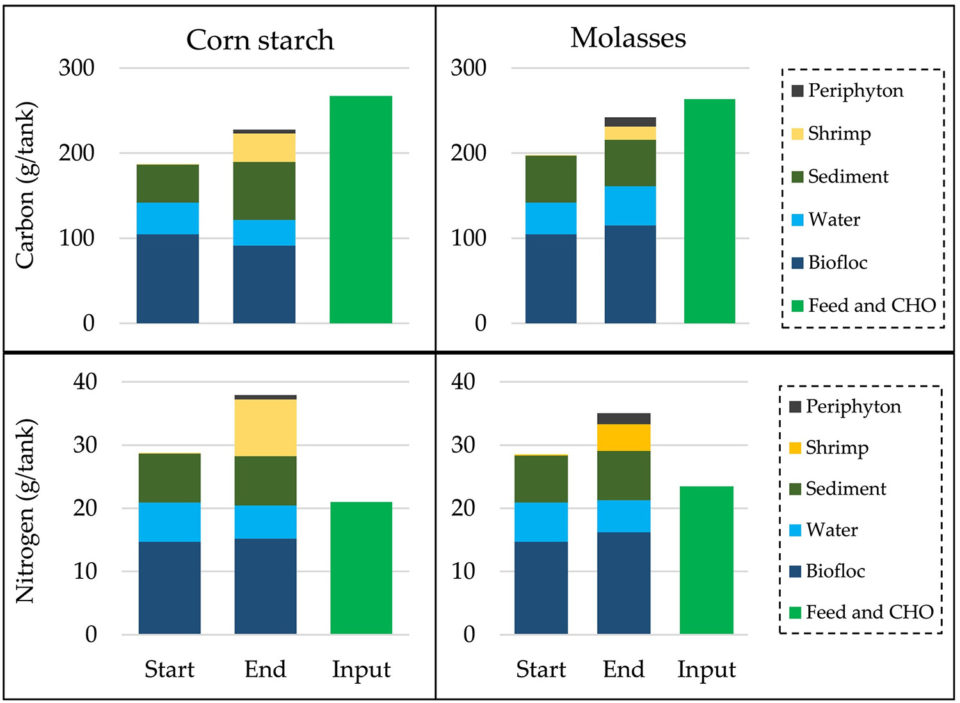
Our data demonstrated that only 15 to 17 percent of carbon and 28 to 43 percent of nitrogen input remained in the system at the end of the experiment. The nitrogen accumulated in shrimp in the corn starch treatment of this research is comparable to that reported by other researchers. However, the total 43 percent of the nitrogen input accumulated in the tank was slightly more than half of the 80 percent accumulation of the nitrogen input reported elsewhere. The other 20 percent was assumed to be lost through denitrification and volatilization. From other researchers working in conventional culture ponds without CHO addition, 23 percent of carbon and 35 percent of nitrogen inputs (i.e. from feed and fertilizer) were reportedly assimilated in shrimp, indicating that our system was less efficient in carbon use compared to more conventional shrimp production systems.
Perspectives
Our results show that the choice of organic carbon source plays an important role in the success of a biofloc system. Corn starch was superior to molasses for enhancing the growth of L. vannamei shrimp juveniles. Once the biofloc is established, nitrogen waste can be efficiently controlled, resulting in relatively little diurnal fluctuation of nitrogen and carbon in culture water. However, the nutrient loss in biofloc systems, especially carbon loss is high, and ways to reduce carbon losses from culture systems should be explored. Also, further research is needed on improving nutrient use efficiency, either directly by the cultured animals or indirectly by trapping nutrients and making them available for other uses.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Tran Huu Tinh, Ph.D.
Aquaculture and Fisheries
Animal Sciences Group
Wageningen University, the Netherlands -
Tom Koppenol, M.Sc.
Aquaculture and Fisheries
Animal Sciences Group
Wageningen University, the Netherlands -
Tran Ngoc Hai, Ph.D.
College of Aquaculture and Fisheries
Can Tho University, Viet Nam -
Johan A.J. Verreth, Ph.D.
Aquaculture and Fisheries, Animal Sciences Group
Wageningen University, the Netherlands -
Marc C.J. Verdegem, Ph.D.
Corresponding author
Aquaculture and Fisheries, Animal Sciences Group
Wageningen University, the Netherlands[108,110,46,114,117,119,64,109,101,103,101,100,114,101,118,46,99,114,97,109]
Tagged With
Related Posts

Aquafeeds
Biofloc and clear-water RAS systems: a comparison
This study compared two types of indoor, shrimp culture systems: clear-water RAS and biofloc systems. Clearwater RAS had the edge in water quality, but shrimp in the biofloc treatment had a higher feed conversion ratio.

Health & Welfare
Comparing biofloc, clear-water and hybrid RAS systems as shrimp nurseries
This study compared three types of RAS nurseries for shrimp: biofloc (BF), clear-water (CW), and hybrid RAS (HY). Results showed that differences between treatments in terms of shrimp survival, mean harvest weight, specific growth rate, and feed conversion ratio were not significant.

Health & Welfare
Recent developments in biofloc technology
Combining biofloc technology with bio-secure modular shrimp culture can make operations more sustainable and economically viable. For optimized biofloc production, lined ponds and reservoirs and high stocking densities are essential. Paddlewheel aerators keep dissolved-oxygen levels high.

Health & Welfare
Studies advance intensive shrimp culture in zero-exchange biofloc raceways
In ongoing trials of greenhouse-enclosed intensive biofloc shrimp culture systems, the objectives of a 2012 study were to evaluate the performance of fast-growth shrimp fed a commercial diet formulated for high-density culture systems, and to further evaluate injector performance in the zero-exchange, super-intensive raceways.


