Results show fish performed better with kelp meal’s higher nutritional content, buoyancy

Biofloc technology (BFT) has emerged as an environmentally friendly and innovative technique to increase efficiency, recycle nutrients, lower water usage and running costs of aquaculture systems, while reducing adverse impacts on the environment. BFT is based on the application to aquaculture environments of standard, wastewater treatment systems.
Biological polymeric substances are required for floc formation, by binding and holding the floc components together. The matrix formed aggregates the microorganisms (bacteria, protozoa and fungi) and facilitates their ingestion by fish, providing direct access to nutrients and acting as a substrate. Each floc remains attached by a matrix of mucus secreted by bacteria, filamentous microorganisms or by electrostatic attraction.
To shorten the start-up period in using BFT, various researchers have discussed the need to investigate the effect of adding nucleation sites to the water at start-up, which will stimulate floc formation. The start-up period could also be shortened by inoculating the water with existing good-performing biofloc or with specific inoculum. Corn and wheat meals are commonly used to stimulate more floc formation because of the starch content, which is a natural coagulant and favors creation of microbial communities around them.
Macroalgae – such as the giant bladder kelp (Macrocystis pyrifera), a brown alga underexploited in northwest Mexico – have not been tested as nucleation sites in floc formation. Kelps have high protein and low carbohydrates contents, can inhibit undesirable bacterial growth and could also serve as good BFT nuclei because of their high content of alginates used as thickener and gelling agents.
This article – adapted and summarized from the original publication [B. Aparicio-Simón et al. 2020. Giant bladder kelp (Macrocystis pyrifera) and maize (Zea mays) meals as nucleation sites for biofloc formation. Aquaculture Reports, Vol.16, March 2020, 100289] – presents the results of a study to evaluate two meals (kelp and maize) as nucleation sites of biofloc formation and test their effect on the performance of Nile tilapia (Oreochromis niloticus) fingerlings.
Study setup
The experiment was carried out for 12 months in indoor, plastic, 150-liter tanks at the Laboratory of Research, Technological Development and Innovation in Tilapia, Nayarit Unit, Centro de Investigaciones Biológicas del Noroeste (UNCIBNOR), in Tepic Nayarit, México. The kelp meal was provided by a commercial supplier and maize meals were bought locally. Both meals were sieved to 400-μm particle size.
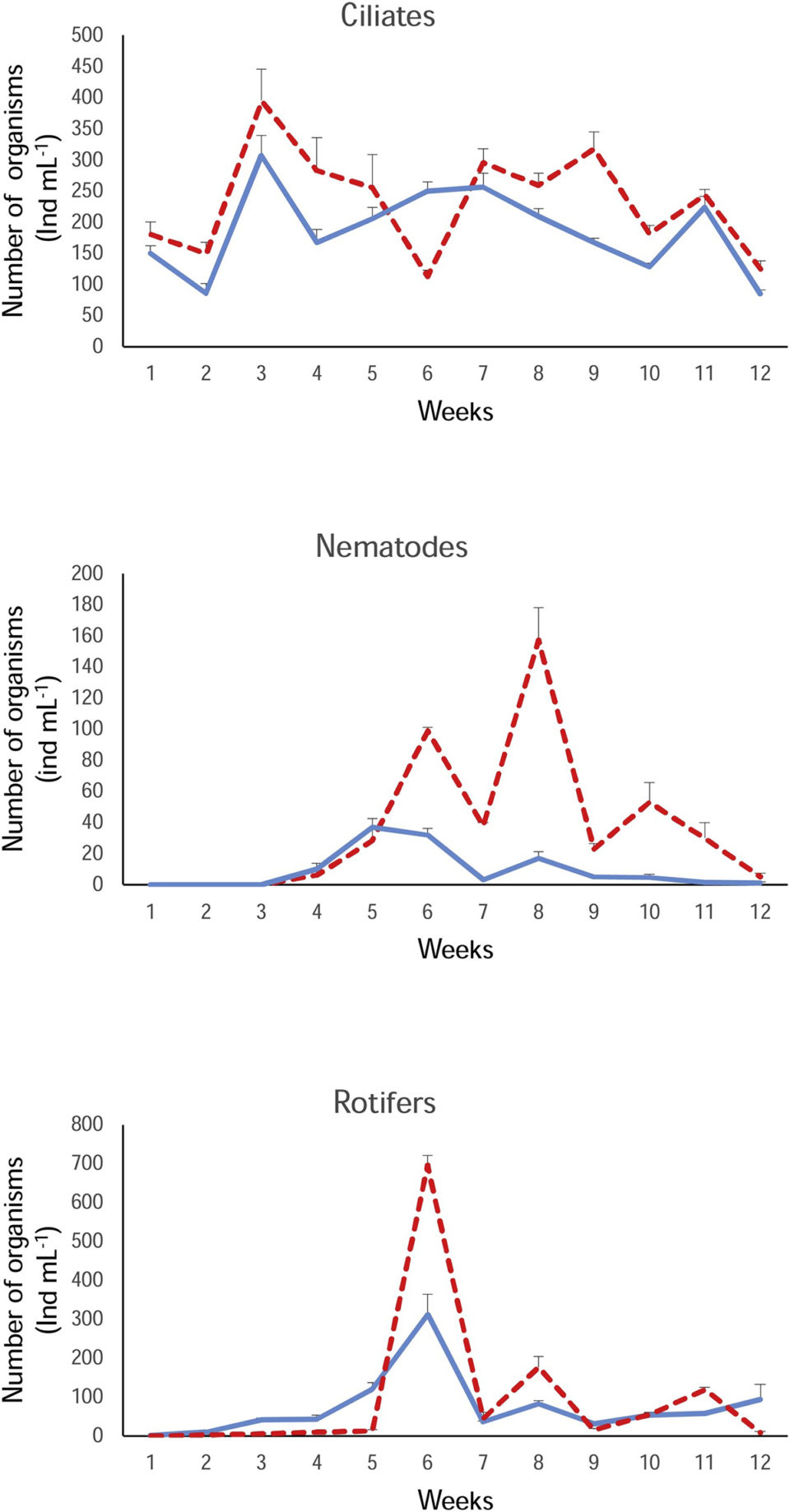
Biofloc was developed in the tanks using a bacterial inoculum and molasses and were left for 24 hours before nucleation site meals were added. Biofloc samples were collected and preserved and their microorganisms were classified into major groups (microalgae, ciliates, annelids, gastrotrichia and rotifers) and were identified at genus level. Ciliates, annelids and rotifers were counted using a microscope.
After four weeks of biofloc induction, 100 O. niloticus fingerlings (1.60 ± 0.02 grams; 4.76 ± 0.03 cm) were stocked into each tank at a density of 1,000 fish per cubic meter. Fish were fed 1 mm pelleted commercial feed (40 percent protein, 15 percent lipid) twice per day 5 percent biomass, and adjusted daily. Molasses was also added to the tanks, as well as kelp and maize which were added daily relative to the desired biofloc volume of 50 mL per liter. Fish were sampled weekly and all fish were harvested at the end of the trial for total biomass determination. Several production parameters were estimated for the tilapia.
For detailed information on the experimental design and conditions; biofloc development and assessment; the Nile tilapia production test; and statistical analyses, refer to the original publication.
Results and discussion
The environmental conditions at which the experiment was performed were similar between treatments and their mean values were within acceptable values for tilapia aquaculture. Water temperature averaged 26 degrees-C, dissolved oxygen concentration remained above 3.54 ± 0.05 mg per liter, also adequate for growth of heterotrophic and nitrifying bacteria. There were no significant differences between ammonium concentration in both treatments, and non-ionized ammonia concentration were similar in both treatments with a mean value of 0.245 mg per liter.
Our results showed that both meals were excellent nucleation sites for biofloc formation. In only a few days, nucleation sites were colonized by microalgae (mainly Chlorophytes), ciliates and rotifers – these microorganisms provide a good source of nutrients to fish, available 24 hours a day. In the case of rotifers, they can fragment floccules and consume the bacteria present, processing and transferring the energy generated to the biofloc, as well as helping to promote the generation of more floccules because of their contribution of mucilage produced by their excreta.
Maize floc had a significantly higher relative abundance of ciliates, annelids and rotifers and it was heavier than the kelp floc. Therefore, we would expect a better performance of the tilapia in this floc. However, our results were the opposite: fish weight and length gain and survival were significantly higher in kelp biofloc, even when considering that fish in the maize floc had some mortality and were raised at a significant lower density from week 7 onwards.
Our results could be due to the particles of kelp floc having apparently higher floatability, and as a consequence, remained in suspension for a longer time and thus readily available for fish consumption. Kelp floc formed a larger plug of spongy consistency in Imhoff cones (devices used for volumetric determination of settleable solids – like floc – in water), and these particles remained longer in suspension even after the 20-minute sedimentation test we used. The spongy consistency may be due to the high alginate content, which is a thickener and gelling agent.
In contrast, the maize floc particles were more compact and formed a smaller plug in the same period of time. Our results concur with those of other researchers reporting that the nutritional value of biofloc to aquatic animals is not dependent only on the ability of the fish to both ingest and digest the particles, but also on the density of suspended particles.
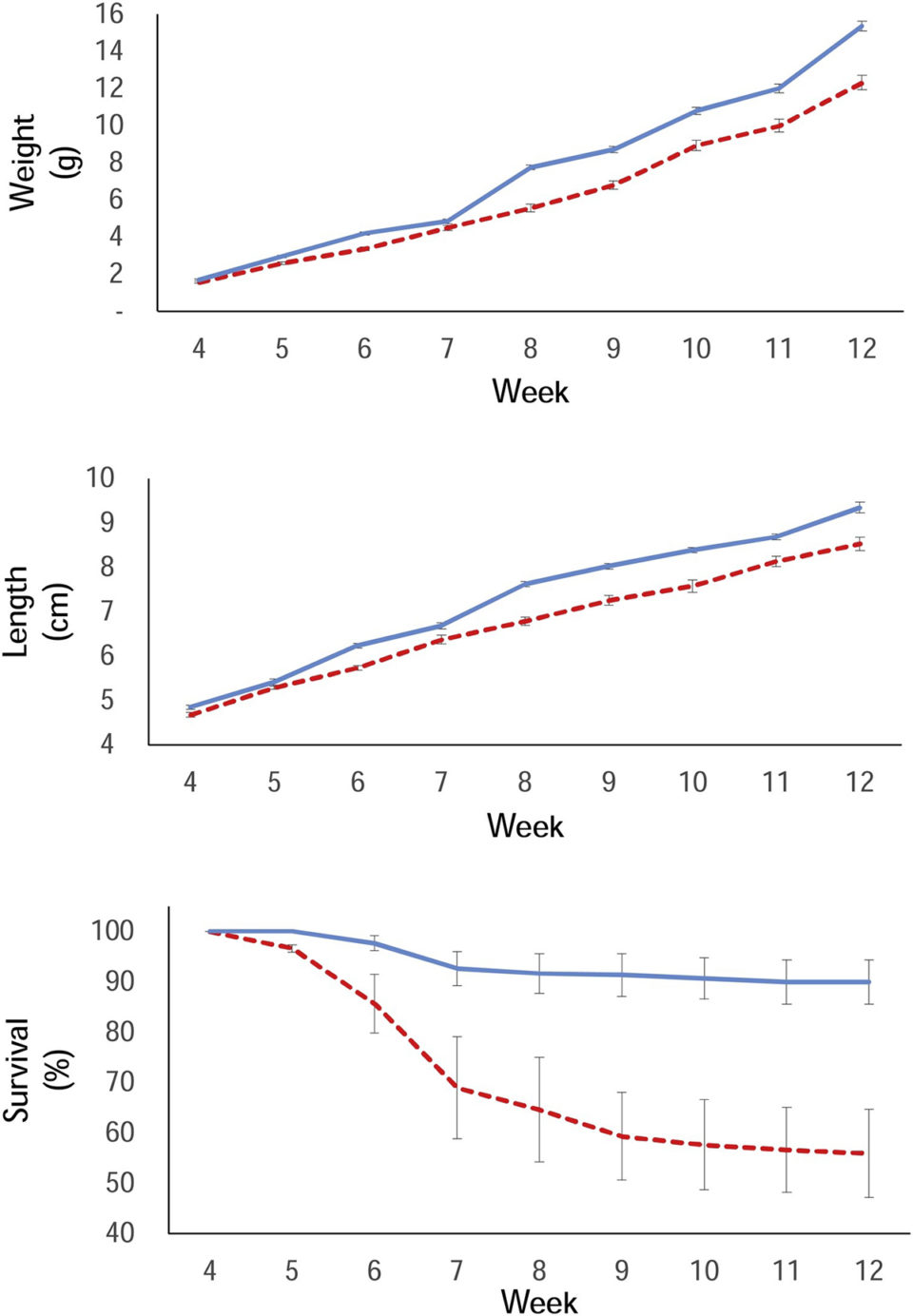
Another factor in our results could be due to differences in the proximal composition of meals. Kelp meal has a higher protein content (9.41 percent) than maize meal (7.35 percent) but lower content of lipids and carbohydrates. Early juvenile fish (0.02 to 10.0 grams) would require a diet higher in protein, lipids, vitamins and minerals and lower in carbohydrates. This could explain the mortalities registered in maize treatment starting in the seventh week of the experiment. Similar results are reported by other researchers.
In addition, M. pyrifera as substrate in a biofloc system has compounds – phenolic, terpenoid and lipophilic with antimicrobial activity – that inhibit undesirable bacterial growth, including pathogens. This bacterial inhibition could be causing a decrease in pathogenic bacteria and promoting the establishment of beneficial denitrifying bacteria.
A study reported that nucleation meal represents only about 0.26 percent of the total production costs in a commercial farm using BFT. But it is likely that the use of M. pyrifera meal is more cost-effective considering the total biomass harvested. In our study, the biomass produced by using M. pyrifera floc was 150 percent higher than the biomass produced by Z. mays floc.
Perspectives
Results of this study showed that both kelp and maize meals can serve as nucleation sites for biofloc formation. However, Nile tilapia fingerlings performed better when using kelp meal as biofloc nuclei than maize meal, probably because of kelp’s higher nutritional (protein) content and higher buoyancy that make these flocs more readily available to the fish.
References available from the original publication.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Benjamín Aparicio-Simón, Ph.D.
CONACYT, Unidad Nayarit del Centro de Investigaciones Biológicas del Noroeste S.C., Calle Dos # 23, Ciudad del Conocimiento, CP 63175, Tepic, Nayarit, Mexico
-
Evelyn Real-Moreno
Planta Experimental de Producción Acuícola, Departamento de Hidrobiología, Universidad Autónoma Metropolitana Iztapalapa, Ciudad de Mexico, Mexico
-
Daniel Espinosa-Chaurand, Ph.D.
CONACYT, Unidad Nayarit del Centro de Investigaciones Biológicas del Noroeste S.C., Calle Dos # 23, Ciudad del Conocimiento, CP 63175, Tepic, Nayarit, Mexico
-
Ricardo García-Morales, Ph.D.
CONACYT, Unidad Nayarit del Centro de Investigaciones Biológicas del Noroeste S.C., Calle Dos # 23, Ciudad del Conocimiento, CP 63175, Tepic, Nayarit, Mexico
-
Rodolfo Garza-Torres, Ph.D.
CONACYT, Unidad Nayarit del Centro de Investigaciones Biológicas del Noroeste S.C., Calle Dos # 23, Ciudad del Conocimiento, CP 63175, Tepic, Nayarit, Mexico
-
Alejandro de Jesús Cortés-Sánchez, Ph.D.
CONACYT, Unidad Nayarit del Centro de Investigaciones Biológicas del Noroeste S.C., Calle Dos # 23, Ciudad del Conocimiento, CP 63175, Tepic, Nayarit, Mexico
-
David Lora-Sanchez
Algas y Extractos del Pacífico Norte AEP, Prolongación Rio Grijalva #1034. CP 22890 Col. Carlos Pacheco, Ensenada B.C, Mexico
-
Alfonso N. Maeda-Martínez, Ph.D.
Corresponding author
Unidad Nayarit del Centro de Investigaciones Biológicas del Noroeste S.C., Calle Dos # 23, Ciudad del Conocimiento, Tepic, Nayarit, 63173, Mexico
Tagged With
Related Posts

Responsibility
Can ranching ‘zombie urchins’ boost uni, save kelp forests?
With Norwegian knowledge and a partnership with Mitsubishi, Urchinomics aims to turn worthless empty urchins into valuable seafood while restoring kelp forests and creating jobs.

Responsibility
Can sustainable mariculture match agriculture’s output?
Global, sustainable mariculture production, developed on a massive, sustainable scale and using just a small fraction of the world’s oceanic areas, could eventually match the output of land-based agriculture production. Scale and international law considerations require the involvement of many stakeholders, including national governments and international organizations.

Aquafeeds
Evaluating brown seaweed supplementation in feed for Atlantic salmon smolts
Study evaluated the effect on Atlantic salmon smolts growth performance when adding abrown seaweed meal (from Laminaria sp., or kelp) to their feed.

Responsibility
Lean and green, what’s not to love about seaweed?
Grown for hundreds of years, seaweed (sugar kelp, specifically) is the fruit of a nascent U.S. aquaculture industry supplying chefs, home cooks and inspiring fresh and frozen food products.


