Low inclusion levels produce acceptable growth, survival, possibly stimulate immune system

More than half of the aquaculture species produced are fed formulated feeds, and several studies have focused on additional terrestrial proteins as ingredient sources – mainly legumes like field peas, lupin and fava beans – that are regionally and organically produced. Legumes provide nitrogen for themselves and subsequent plants and thus reduce the overall fertilization needs during crop rotation.
Several different cultivars of lupin have been tested with different aquaculture species and with generally promising results. Lupin digestibility has been shown to exceed that of soy in Atlantic salmon. Extruded lupin seed meal has been found to offer good digestibility coefficients, especially for protein and energy, in trout and turbot, and can greatly substitute fishmeal in European seabass diets. At least 40 percent of fishmeal protein in diets of the black tiger shrimp (Penaeus monodon) can be replaced by lupin kernel meal (used on a protein‐equivalent basis), without adverse impacts on growth. And a study showed that the Andean lupin (Lupinus mutabilis) can replace at least half of the fishmeal protein – equivalent to a third of the total protein – in the diet of Pacific white shrimp (Litopenaeus vannamei) without negatively influencing growth and feed conversion. However, information about the effects of metabolic and immune parameters is missing so far.
This article – adapted and summarized from the original publication [M. Weiss et al. 2020. Lupin kernel meal as fishmeal replacement in formulated feeds for the Whiteleg Shrimp (Litopenaeus vannamei). Aquaculture Nutrition 2020;00:1–11.] – reports on a study to evaluate narrowleaf lupin (Lupinus angustifolius) seed meal as a sustainable diet component for Pacific white shrimp in controlled feeding experiments conducted in a recirculating aquaculture system.
This study is part of the project “TRansition paths to sUstainable legume‐based systems in Europe” (TRUE), and it has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 727973. The authors thank the staff from the “Centre for Aquaculture Research” (ZAF) for conducting water quality analyses and daily routine activities.
Study setup
The study was conducted in a recirculating aquaculture system (RAS) system at the Center for Aquaculture Research, Alfred Wegener Institute in Bremerhaven, Germany. The setup included 18 separate tanks with a water treatment system including a mechanical filter, a protein skimmer, a biofilter and ozone treatment. During the trial, physical water parameters were measured every day (91.93 ± 4.95 percent for dissolved oxygen; 7.53 ± 0.11 for pH; 26.11 ± 0.69 degrees-C for temperature and 15.96 ± 0.53 grams per L for salinity). Twice a week, the concentrations of nitrogen compounds were determined, with mean concentrations of 0.27 ± 0.245 mg/L for ammonium, 2.25 ± 3.692 mg/L for nitrate and 122.71 ± 96.16 mg/L for nitrite.
L. vannamei postlarvae (PL13, mean weight ~ 3 mg ± 0.5 mg, mean ± standard deviation) were obtained from a commercial shrimp hatchery in Florida (USA) and grown for seven weeks. Tanks were each stocked with 25 individuals at a mean biomass per unit of 90.22 ± 0.86 grams. Controlled feeding was maintained for eight weeks and treatments were done in quadruplicate. Weight and length gains were recorded at the beginning, and after four and eight weeks. At the end of the experiment, haemolymph samples were collected for further analyses, after determining the molting stage of each animal and excluding freshly molted animals due to known impacts of molting on various metabolic and immune parameters.
Four experimental, isonitrogenous and isocaloric, diets were formulated to meet the requirements of L. vannamei during the study, considering energy content, protein and amino acid profile, lipid and fatty acid composition, vitamins and minerals. As necessary, single amino acids (methionine and lysine) were added to balance the amino acid profile. The diets included a control with fishmeal as main protein source; diets L10 and L20 with 10 and 20 percent, respectively, lupin kernel meal replacing fishmeal; and L30 with 30 percent lupin kernel meal in complete replacement of fishmeal. A commercial shrimp diet (Beeskow, Germany; protein 390 grams per kg, lipid 90 grams per kg, ash 90 grams per kg, fiber 15 grams per kg) without lupin meal was tested in duplicate for comparison.
For detailed information on the experimental system and diets; phenoloxidase activity; total and differential haemocyte counts; and statistical analyses, refer to the original publication.
Results and discussion
All animals accepted the feeds and showed acceptable growth. The average survival rate of shrimp across all lupin meal supplemented diets was 68.3 ± 7.3 percent. For the animals receiving the control diet, it was 63.0 ± 5.0 percent, and survival rates were not statistically different between treatments. After eight weeks, the body weight of the animals differed significantly depending on the diet (Table 1). The animals fed the control feed, the L10 feed and the commercial diet were the heaviest. Shrimp fed the L20 diet were significantly lighter than control and L10 diet but did not differ from the commercial diet. Shrimp fed the L30 diets had significantly lower body weights than all other treatments. These findings are also reflected in the specific growth rate, which is above 1.5 for all diets but drops below 1.0 in shrimp fed the L30 diet. The reduced growth trend in the L30 treatment was already evident after four weeks.
Weiss, Lupin meal, Table 1
| Diet | Average biomass, start (g) | Average biomass, end (g) | Weight gain (g) | Specific growth rate (% BW/day)* |
|---|
Diet | Average biomass, start (g) | Average biomass, end (g) | Weight gain (g) | Specific growth rate (% BW/day)* |
|---|---|---|---|---|
| Com | 3.60 ± 0.00 | 9.50 ± 0.55 | 5.898 | 1.731 |
| Control | 3.62 ± 0.05 | 10.37 ± 0.55 | 6.747 | 1.878 |
| L10 | 3.61 ± 0.05 | 9.66 ± 0.58 | 6.044 | 1.756 |
| L20 | 3.61 ± 0.02 | 8.53 ± 0.55 | 4.924 | 1.537 |
| L30 | 3.60 ± 0.03 | 6.24 ± 0.61 | 2.644 | 0.984 |
Table 1. Growth results of the feeding experiments for initial weight, weight gain and specific growth rate of L. vannamei. Replicate numbers were 24, 63, 73, 73 and 59 for Com, control, L10, L20 and L30, respectively. *Percent body weight per day.
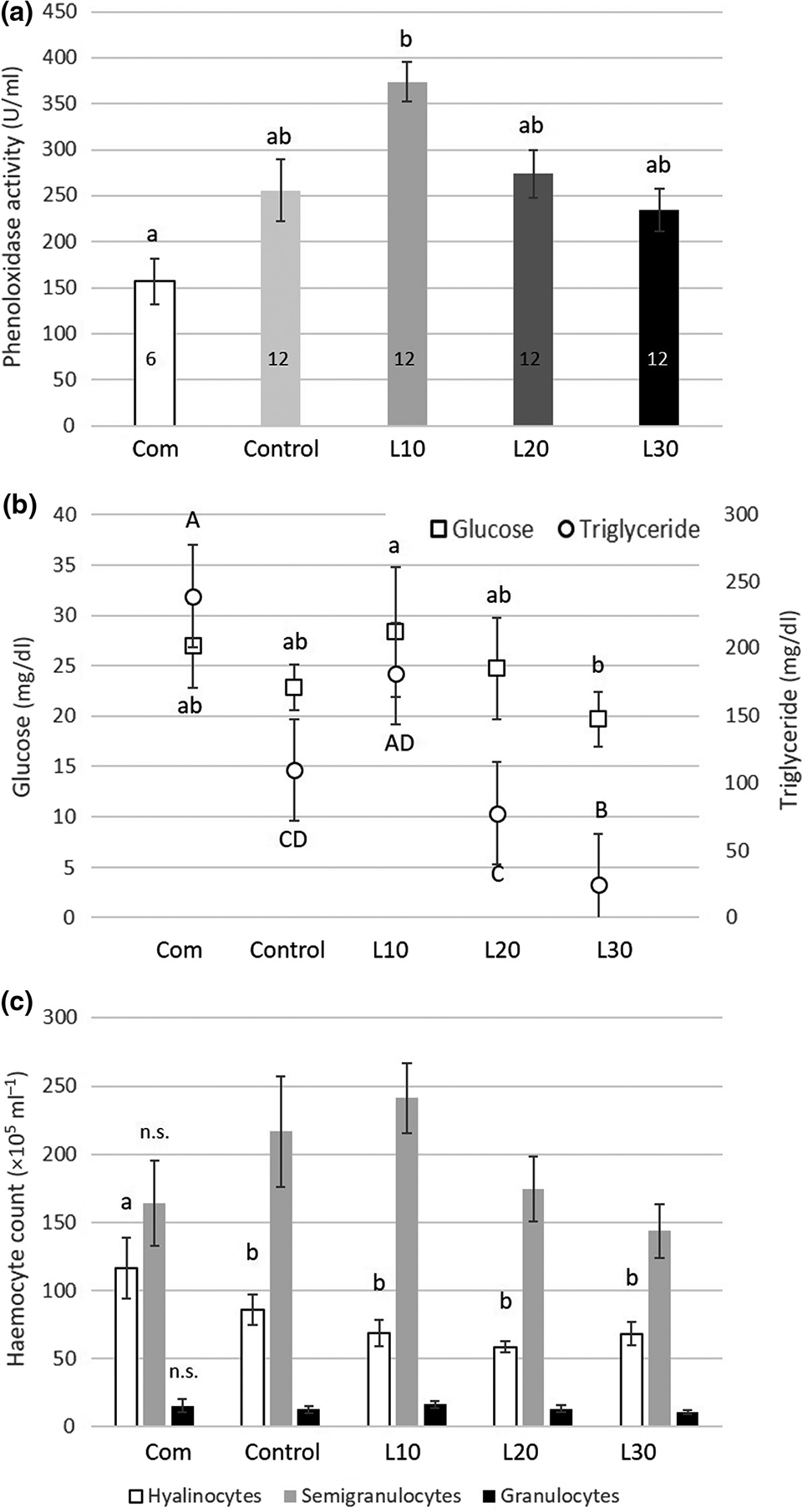
The haemolymph phenoloxidase (PO) activity [an important component of the shrimp immune defense] was highest in animals fed the L10 diet and significantly lower when fed the commercial diet (Fig. 1a). Activity in the control and the L20 and L30 diets was intermediate, without significant differences compared with the commercial (Com) or the L10 diet.
The glucose level was significantly higher in L10 fed animals (28.36 ± 6.44 mg/dl) than in L30 fed animals (19.71 ± 2.73 mg/dl) (Fig. 1b). Glucose levels of shrimp fed the commercial feed (27.0 ± 4.15 mg/dl), the control feed (22.89 ± 2.26 mg/dl) and L20 (24.73 ± 5.0 mg/dl) were intermediate and did not differ significantly from L10 or L30.
The mean total haemocyte count (THC) values were 277.6 ± 118.7 ×105 cells ml/L. Total haemocytes were higher when shrimp were fed low levels of lupin (L10) than the THC values of shrimp fed the commercial and the control feed. THC values gradually decline with higher lupin inclusion levels. No significant differences between the dietary treatments occurred. However, we observed a tendency that semi-granular cells are promoted in animals fed the L10 diet but are reduced with further increasing lupin content in the feed (Fig. 1c).
Sustainable and viable alternative proteins remain high priorities for future aquaculture development. The results of our study clearly indicate the applicability of lupin seed meal as a replacement for fishmeal in diets for L. vannamei culture. Inclusion is, as with many terrestrial alternative sources, recommendable but only within clear limitations. The growth of L. vannamei indicates that increasing inclusion rates of lupin meal exceeding 100 g/kg (replacing 40 percent of fishmeal) in the feed cause a progressive decline in shrimp performance.
New ingredients for aquafeeds, especially plant‐derived products, can have impacts on the metabolism of the animal that might not be expressed on the growth level but in metabolic parameters. The metabolic data from our study show that while inclusion of 10 percent lupin meal has no negative influence, increasing supplementation with lupin meal (20 and 30 percent) progressively deteriorates the physiological status of L. vannamei shrimp towards lower metabolite content in the total haemolymph.
In general, total protein levels in the haemolymph of shrimps fed the different diets are in the range reported for animals maintained under similar conditions. Our results show low but stable haemolymph protein levels for all diets, indicating a limited but sufficient protein supply not affected by the rate of lupin meal inclusion in the diets tested.
Our results also provide evidence that the inclusion of lupin meal in the feed has a modulating effect on the shrimp’s immune system with positive enhancement of the haemocytes and the phenoloxidase (PO) system when the lupin is included in moderate levels (10 percent; Fig. 1a,c). Most of the designed diets resulted in slightly higher PO activity, but the L10 diet‐treated animals showed a significant increase in PO activity compared with the commercial diet. Higher values of PO activity were also found by various other authors in L. vannamei when other immune‐stimulating and probiotic ingredients were included in the feed.
Perspectives
Our results demonstrate the successful inclusion of dehulled lupin seed meal in feeds for L. vannamei without adverse effects on survival, growth performance or metabolic parameters for inclusion rates of up to 100 grams per kg of feed. High inclusion rates (300 grams per kg lupin seed meal) resulted in reduced growth performance and nutritional status. An immune‐stimulating effect on the shrimp was detected for an inclusion level of 100 grams per kg lupin meal, based on an increase in phenoloxidase activity.
These results prove that dehulled lupin seed meal is a suitable, regional alternative protein source for aquaculture feeds that can supply good quality protein to L. vannamei shrimp and can replace significant amounts of diet fishmeal. For future diet developments, higher substitution rates might be achieved by supplementing a mix of lupin meal and other regional plants, such as fava bean. This might provide a more balanced nutritional supply and make use of the immuno‐stimulating effect of moderate lupin inclusion rates. Additionally, further research is required to assess methods for lupin pretreatment to enhance digestibility.
References available from original publication.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Monika Weiss
Corresponding author
Helmholtz Centre for Polar and Marine Research
Alfred Wegener Institute
Bremerhaven, Germany -
Anja Rebelein
Helmholtz Centre for Polar and Marine Research
Alfred Wegener Institute
Bremerhaven, Germany; and
Thünen Institute for Fisheries Ecology
Bremerhaven, Germany -
Matthew J. Slater
Helmholtz Centre for Polar and Marine Research
Alfred Wegener Institute
Bremerhaven, Germany
Tagged With
Related Posts

Aquafeeds
Solid-state fermentation novel process for improving nutritional value of plant feedstuffs
Solid-state microbial fermentation for upgrading nutritional characteristics of raw plant materials is a potential pretreatment for animal feeds.

Aquafeeds
A push for rapeseed as a viable aquafeed ingredient
One Germany-based company says rapeseed protein concentrate, or RPC, can help aquafeed manufacturers meet growing demand.

Aquafeeds
Canada eagerly looks to camelina oil as a fish oil alternative
Grown worldwide, Camelina sativa is a tiny seed used for human consumption that also has big potential for driving the “green” economy.

Health & Welfare
High-soy, fishmeal-free diets support Florida pompano growth
The authors have achieved good growth using a fishmeal-free diet for the carnivorous marine fish Florida pompano. In studies, they used pompano as a model marine species in a cost/benefit analysis of two extruded diets.


