Results showed improved growth and survival, including for animals challenged with AHPND

The Pacific white shrimp (Litopenaeus vannamei) is an important aquaculture species globally. The species has high production and productivity of its high tolerance to environmental changes, and fast growth in intensive and super-intensive culture systems with high survival rates. Production of good quality L. vannamei postlarvae (PL) is increasingly important due to high market demand, and there are various challenges including management of production environments, various important diseases, and dietary nutrients.
In addition to genetic improvement programs, L. vannamei shrimp can be nourished strategically during its larval development to better meet quality indices like the metamorphosis index (the measure of larval shrimp development from Zoea to PL), larval survival rates and their overall resistance against diseases and environmental stressors.
Previous studies have shown that various vitamins can improve the shrimp’s immune system, flesh quality, growth and survival rates, and resistance against various diseases and stressors. For example, vitamins A and C contribute to a shrimp’s tolerance to less-than-ideal environmental conditions and also improve body structure, growth, immunity and sexual maturation. Vitamin E is believed to be important as a metabolic antioxidant due to high inclusion of polyunsaturated fatty acids in crustacean diets. Also, essential amino acids like methionine must be provided from external sources given the ongoing trend of replacing fishmeal with plant-based proteins.
During the early development stages, shrimp larva and juvenile are sensitive and require a high level of diverse nutrients. Vitamins and amino acids are essential but need to be supplemented to the larvae or provided in their aquafeeds. But aquafeed manufacturing processes (extrusion/pelleting) involve high temperatures and may degrade essential nutrients, particularly in small-size feeds.
Here we report on the results of our research on the supplementation of some of these nutrients through the use of a commercial product, Nutrimix (a vitamin and amino acid complex manufactured by Virbac Vietnam) and combined immersion and oral application strategies on L. vannamei larvae and juveniles, with the goal of improving the production of better-quality PLs for the shrimp aquaculture industry.
https://www.aquaculturealliance.org/advocate/ahpnd-is-a-chronic-disease-in-pacific-white-shrimp-from-latin-america/
Experimental setup
The study was carried out at the experimental hatchery of the College of Aquaculture & Fisheries, Can Tho University, Vietnam. Larval L. vannamei shrimp were reared in 500-liter indoor tanks during three separate experimental phases. Experimental diets with inclusions of the commercial additive Nutrimix were applied via two routes: by immersion (from Zoea-1 to PL-12) and feeding (from PL-12 to PL-42) based on the different developmental phases of the shrimp. All experimental treatments were performed in triplicate.
In the study we evaluated the effect of the additive on the growth and development of L. vannamei PLs and their survival rate during a three-phase study. We also assessed mortality on experimental shrimp caused by a challenge with Acute Hepatopancreatic Necrosis Disease (AHPND) with Vibrio parahaemolyticus. Water quality parameters were measured during the study to ensure their maintenance within suitable ranges for shrimp culture.
Phase 1
Three groups of shrimp were used with three replicates per treatment: treatments included the commercial additive at 5 grams per cubic meter per day and 3 g/m3/day, and without the commercial additive (control) during 18 days at 30 ppt salinity from Zoea-1 to PL-12. The indoor tanks were initially stocked at 100,000 nauplii per tank. Water quality parameters were recorded regularly: water temperature and pH were measured every day, and total ammonia-nitrogen (TAN), nitrite and alkalinity were measured every three days.
Data evaluation of this phase was based on the metamorphosis index, survival rate percent of PL-12, and larval length at the end of the Zoea-3, Mysis-3, PL-5 and PL-12 stages. Additional stress tests (with 30 PLs in each test) were done in triplicate with exposure to 100 ppm formalin for 30 min, and 50 percent salinity reductions (from 30 ppt to 15 ppt) for 30 min.

Phase 2
Shrimp from each of the treatments from phase 1 were divided into two groups of 1,000 individuals each. A total of six treatments (including the control) were used, with Nutrimix added to feed pellets at 15 grams per kg of feed for the next 30 days, from PL-12 to PL-42. All treatments were performed in triplicate at a stocking density of 1,000 PLs per tank and at 15 ppt salinity. Shrimp were fed four times per day manually to satiation for 30 days. Water quality parameters were recorded regularly: temperature and pH were measured every day, and TAN and nitrite were measured once per week. Growth parameters such as weight gain (WG), daily weight gain (DWG), specific growth rate (SGR) and survival rate (SR) were collected and analyzed for this phase for comparison between treatments.
Phase 3
The remaining shrimp after the conclusion of phase 2 were used in phase 3. An immersion, Acute Hepatopancreatic Necrosis Disease (AHPND) challenge was carried out with 30 shrimp from each experimental group (six groups in total, same as in phase 2; Table 1) with a Vibrio parahaemolyticus isolated in the Mekong Delta. An immersion dose of 1.9 × 104colony forming units, CFU per mL was used, and mortality assessments were done for 14 days after the challenge (post-challenge period).
[table “1540” not found /]
Statistical analyses of the data collected included one way ANOVA and Tukey’s post-hoc tests using Minitab version 19 to compare data of all treatments obtained from the three phases of the study.
Results and discussion
Water quality
Water temperature varied from 28.0 to 30.9 degrees-C and pH was maintained at ~8.1 during the durations of phases 1 and 2. For nitrogenous compounds in phase 1, TAN and nitrite, fluctuated in the range of 0.56 to 0.71 and 0.14 to 0.18 mg per liter, respectively, while alkalinity ranged from 150.0 to 152.1 mg calcium carbonate CaCO3 per liter during the 18-day period. For phase 2, recorded TAN ranged from 0.38 to 0.63 mg per liter, and nitrite fluctuated in the range of 0.38 to 0.49 mg per liter. These values were within suitable ranges for normal development of L. vannamei shrimp.
Phase 1 – Development of larvae and survival rate of PL-12
Metamorphosis index (MI). Samples taken on days 3 and 6 showed no differences among treatments. Starting on day 9, the MI was different among treatments, i.e., immersion with the additive at 5 and 3 g/m3/day produced 100 percent of PL-2 compared to 100 percent of PL-1 in the control. On the last day of phase 1, immersion treatments (both 5 and 3 g/m3/day) produced 100 percent of PL-11 in comparison with 100 percent of PL-10 in the control.
Length of larvae and PL (mm). The length of shrimp larvae among treatments was not significantly different (p>0.05), but the addition of 5 g/m3/day of the additive produced the best results at the PL-5 stage compared to the control (p<0.05). Additionally, the results at the PL-12 stage showed significant differences in length between supplemented treatments (both at 5 and 3 g/m3/day) and the control after the experimental period of 18 days. This research is among the very first studies to investigate the effect of blended vitamins and amino acids supplemented in the rearing water of the shrimp larval stages from Zoea-1 to PL-12. Our results showed the benefit of the supplementation on the metamorphosis index and the survival rates of the experimental shrimp.
Survival rate to PL-12. The survival data of PL-12 presented in Table 2 showed the highest rate at a Nutrimix dose of 5 g/m3/day and then at 3 g/m3/day, significantly different from the control treatment. Additional stress tests on surviving shrimp showed better results for the 5-gram treatment tested with exposure to 100 ppm formalin, and 100 percent survival of all treatments with exposure to 15 ppt salinity.
[table “1541” not found /]
Phase 2 – Growth of PL fed with a vitamin and amino acid complex
Growth performance of PL. The initial mean body weight of the experimental shrimp was ~0.007 g/ind. Feeding with and without the additive for 30 days showed significant differences on final body weight (FBW), WG, DWG and SGR (p<0.05) (Table 3). Our data suggest that the continuous addition of the commercial additive from Zoea to PL could enhance shrimp growth, particularly at the inclusion range of 5g-15g. The survival rate for PL-42 was highest in the 5g-15g and 3g-15g treatments (~93 percent, p>0.05) after 30-days, which is statistically different from the other treatments (p<0.05).
[table “1542” not found /]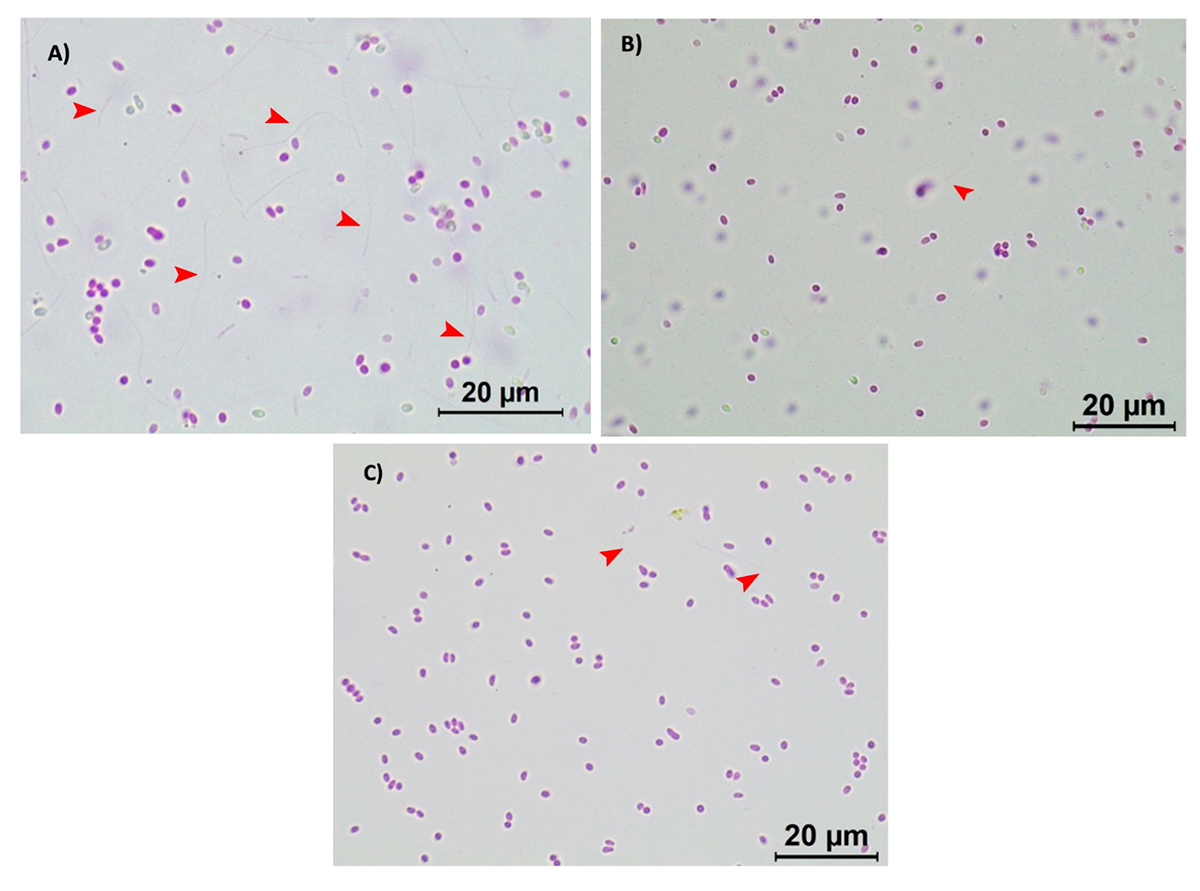
Phase 3 – AHPND challenge test
The AHPND challenge test using a local strain of V. parahaemolyticus reached 70 percent mortality after seven days of post challenge (Control 0g-0g, Fig. 3) with confirmation of clinical signs (Fig. 4) and molecular tests. During the post-challenge period, the shrimp were fed non-supplemented feed in all treatments. The cumulative mortality of shrimp in the additive-supplemented treatments were significantly lower than the positive Control 0g-0g treatment (p<0.05, Table 4).
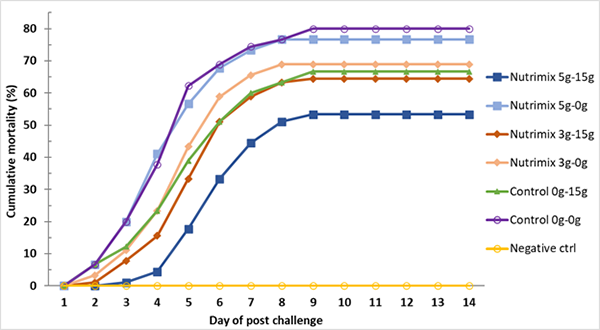
Based on the resulting data, it appears that L. vannamei from PL-12 to PL-42 fed with the Nutrimix additive could be protected from AHPND infection at up to 34 percent when compared to the control, and that combining additive application by immersion and by feeding could also increase the duration of additive retention by up to three days before dead shrimp were observed in the 5g-15g additive treatment.
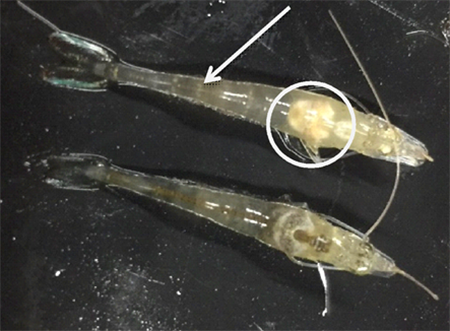
During the larval stages of aquacultured species, several biological functions (e.g., skeletal development, growth and survival) as well as physiological functions (e.g., resistance to toxicants and stress, and immunoactivity) might be enhanced by supplemental dietary vitamins and amino acids. Previous publications have shown the ability of stress reduction on aquatic animals with the dietary inclusion of high levels of some vitamins. For example, vitamin C added to the diet of L. vannamei is well documented to increase resistance of shrimp PLs against stressful environmental conditions and bacterial infections. Our results demonstrated that the added blended complex of vitamins and amino acids in the additive we evaluated in our study can also improve growth, health and survival of young L. vannamei challenged with AHPND.
[table “1543” not found /]Perspectives
Our study results show that L. vannamei growth and metamorphosis indexes were positively improved by adding Nutrimix in the water used to raise Zoea to PL at both treatment rates of 5 and 3 g/m3/day. The additive also significantly improved the survival rate of shrimp compared to the control treatment which did not receive the additive. The beneficial effect of the additive was further enhanced by supplementing it in the diet of PL-12 to PL-42 at a dose of 15 grams per kg of feed.
The AHPND challenge by a V. parahaemolyticus strain resulted in high mortality to the control treatment, but shrimp in the additive-supplemented treatments survived significantly better after the post-infection period of 14 days. The data indicate that a longer feeding application of the additive extended to the post-challenge period may further improve the survival of AHPND-challenged L. vannamei.
Overall, our results of the new application strategy we used for Nutrimix, combining immersion and feeding introduced during the shrimp rearing from Zoea to PL-42, can result in better growth performance and survival rate of young L. vannamei shrimp in challenging conditions.
References available from the corresponding author.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Phuong Do, M.Sc.
Virbac, Aquaculture Division, France and Vietnam
-
Tao Tai Chau, Ph.D.
Can Tho University, Vietnam
-
Philippe Mahl, Ph.D.
Virbac, Aquaculture Division, France and Vietnam
-
Hoang Phan, Ph.D.
Corresponding author
Virbac, Aquaculture Division, France and Vietnam


