Results show isoquinoline alkaloids from Macleaya cordata can be used as feed additives to support farmed shrimp health, resistance to Vibrio infection

Natural products from plants are becoming increasingly utilized as feed additives in the production of terrestrial and aquatic species. Medicinal plants usually possess multiple biological activities – such as antimicrobial, anti-inflammatory, antioxidant, immunostimulatory and appetite-stimulating effects – that could potentially enhance the growth performance and health condition of animals.
Plant-derived isoquinoline alkaloids (IQs) are natural active components of various plants including the pink plume poppy (Macleaya cordata), an herbaceous perennial plant in the family Papaveraceae and widely distributed in China. It has long been used in traditional Chinese medicine as a topical agent for the treatment of inflammation and certain skin diseases.
The main bioactive isoquinoline alkaloids, namely sanguinarine and chelerythrine, are well known for their anti-inflammatory and antimicrobial properties. Currently, IQs are considered promising feed additives to improve overall health condition and replace unnecessary antibiotic use in animal farming. The potential health-promoting properties of IQ from M. cordata have been demonstrated in many animal species, including swine, chicken and fish. These effects include growth performance enhancement, increased appetite, immunostimulation, alteration of the gut microbiome and increased resistance to bacterial infection.
Regarding the application of IQs in shrimp aquaculture, our previous study revealed that Pacific white shrimp (Litopenaeus vannamei) fed IQs (100–200 mg/kg feed for 60 days and challenged with Vibrio harveyi) had a lower intestinal Vibrio spp. count compared to control shrimp, even though the effects on growth and survival were not significant. We suspected that the limited effectiveness of the IQ extracts in promoting the growth and survival rate of the shrimp in our previous study might be related to the methodology of experimental diet preparation. Thus, the experimental feeds in the current study were made by mixing the IQ extracts with the other ingredients before pelleting to minimize the leaching problem of the IQs, and we expected the health-promoting activity of the IQ extracts on L. vannamei shrimp in the present study would be more prominent.
This article – adapted and summarized from the original publication [Bussabong, P. et al. 2021. Effects of isoquinoline alkaloids from Macleaya cordata on growth performance, survival, immune response and resistance to Vibrio parahaemolyticus infection of Pacific white shrimp (Litopenaeus vannamei)] – reports on the results of a study that investigated under laboratory conditions the effects of two different commercial formulations of IQs, which contained the IQ sanguinarine as the main active compound at the concentrations of 0.5 and 1, on the growth performance, survival rate, immune response and resistance to Vibrio parahaemolyticus infection of Pacific white shrimp.
https://www.aquaculturealliance.org/advocate/gamma-irradiation-enhances-nutritional-value-of-animal-by-products/
Study setup
The study was divided into two experiments. In Experiment 1, the effects of IQs on growth, survival and the immune system were investigated in healthy shrimp. In Experiment 2, the effects of IQs on shrimp growth, survival, and resistance to V. parahaemolyticus infection were evaluated after challenge via immersion.
For Experiment 1, five experimental diets were formulated: commercial pelleted feed without IQ supplementation (control diet), feed supplemented with a preparation of commercial, powdered IQ (IQ-E; Sangrovit® Extra) at 200 or 300 mg/kg of feed (providing 1 or 1.5 mg sanguinarine per kg of feed, respectively) and feed supplemented with a commercial, water-soluble, granulated preparation of IQ (IQ-WS; Sangrovit® WS) at 100 or 150 mg/kg of feed (providing 1 or 1.5 mg sanguinarine per kg of feed, respectively).
Pacific white shrimp postlarvae 9 (PL-9) were obtained from a commercial shrimp hatchery in Chachoengsao Province, Thailand, and transported to the Aquaculture Business Research Center (ABRC) laboratory at the Faculty of Fisheries, Kasetsart University, Thailand. Following three days of acclimation, 2,000 PL-12 shrimp (about 1.5 mg/PL) were randomly distributed into 20 fiberglass tanks (five groups with four replicates/treatment group and 100 shrimp/tank), resulting in approximately 150 shrimp per square meter. Throughout the study period, water quality parameters were maintained within suitable ranges.
In Experiment 1, shrimp were fed ad libitum with one of the five experimental diets four times per day for 30 days. On days 10, 20 and 30 of the feeding trial, 10 shrimp per tank were randomly selected and weighed. The shrimp were weighed individually and survival rates in each tank were determined at the same time.
After completion of Experiment 1, the surviving shrimp (about 1.3 grams) in the four IQ groups were transferred into new 16 fiberglass tanks (four replicates/treatment group and 30 shrimp/tank). Shrimp from the control group in experiment 1 were randomly divided into two new groups: positive control (challenged with V. parahaemolyticus) and negative control (without V. parahaemolyticus inoculation), for a total of 24 tanks (six groups with four replicates/treatment group) used in Experiment 2. The animal husbandry and water quality parameters of Experiment 2 were similar to those of Experiment 1 throughout the study period.
For detailed information on the experimental design, animal husbandry and preparation of diets; the immersion challenge with V. parahaemolyticus; data collection and analyses; and statistical analyses, refer to the original publication.
Results and discussion
The isoquinoline alkaloid formulations tested in the present study contained 0.5 and 1 percent sanguinarine, respectively. When incorporated into the pellet feeds, the shrimp diets contained sanguinarine at doses of 1 or 1.5 mg/kg of feed. Since sanguinarine and other alkaloids found in M. cordata have antimicrobial and anti-inflammatory activities, it can potentially be used as a growth promoter. In fact, many previous studies have demonstrated the growth-stimulating effect of IQs in a variety of animal species including swine, chicken and various fishes. In line with these studies, our results revealed growth-enhancing effects of the four IQ-supplemented diets.
It is relevant mentioning that only the V. parahaemolyticus-infected shrimp fed IQs (in Experiment 2) showed a significant improvement in body weight compared to the control group, whereas the healthy shrimp (in Experiment 1) did not. These observations support the antimicrobial and anti-inflammatory hypotheses mentioned above as the main mechanisms for the growth-promoting effect of IQs.
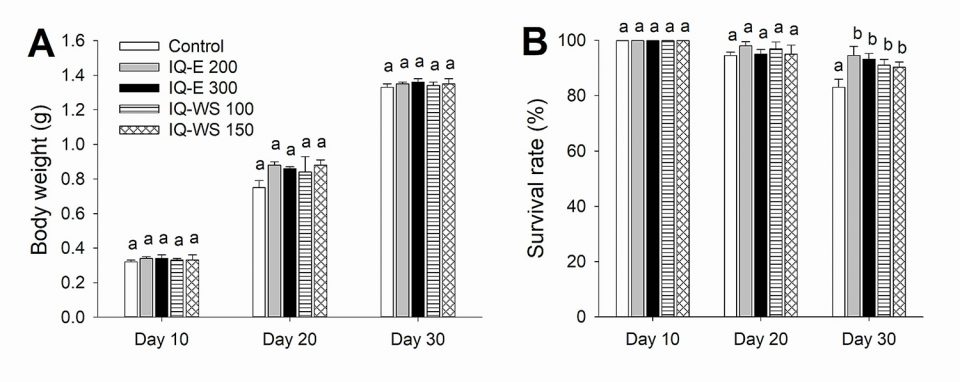
In contrast to this present study, our previous research did not show the improved growth performance and survival rates of shrimp that were administered IQ-WS at 100 and 200 mg/kg of feed diets. A possible explanation for the previous ineffective outcomes may be related to the different methods of experimental feed preparation. While the preparations were mixed with the feed ingredients prior to pelleting in the present study, the previous experiment employed a top-coating method without adding an oil binder which might result in significant leaching of IQs from the prepared diets, thereby rendering the administered doses of the IQ lower than expected. Therefore, to prevent leaching and obtain the best result, IQ preparations should be incorporated into shrimp feed mash before making the pellet feed instead of utilizing a simple top-dressing method.
Herbal extracts have long been studied as enhancers of the immune functions of aquatic animals with promising results, even though the exact molecular mechanisms are often unknown. The effects of plant immunostimulants usually include stimulation of phagocytosis, as well as increased nonspecific immunity mediators.
Our results demonstrated the immunostimulatory effect of IQs on several immune parameters of the experimental shrimp, including total hemocyte count; phagocytic, phenoloxidase and others. Similarly, IQ-supplemented diets have been reported to enhance various immune functions in other cultured aquatic species like red tilapia, common carp and koi carp, and also enhance antibody production in chickens and swine. These improved immune responses may have partly contributed to the higher survival rates of the IQ-fed shrimp in our experiments.
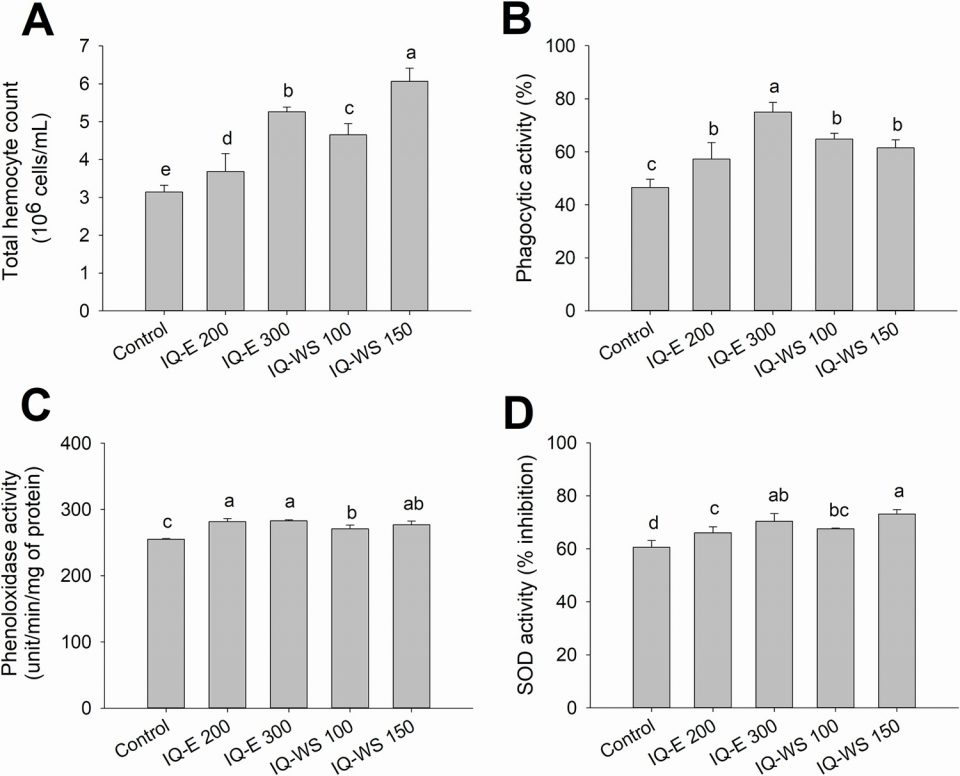
In addition to enhanced immune function, the higher survival rates of the shrimp could be attributed to the antimicrobial activity of the active compound sanguinarine. Sanguinarine has been found to affect the bacterial cell membrane and interfere with bacterial cell division.
Although the direct evidence of antimicrobial activity of sanguinarine or IQ extracts against shrimp pathogens has yet to be reported so far, it is most likely that sanguinarine or IQ extracts may also exert such an effect. Our previous work revealed a significant reduction of the intestinal Vibrio spp. count of V. harveyi-infected shrimp that were fed IQ-WS at 100 and 200 mg/kg of feed, although the survival rates were not significantly improved. However, the shrimp that were fed IQ-supplemented diets in the present study showed better resistance to V. parahaemolyticus infection, as suggested by the higher survival rates of all IQ-fed groups compared to the V. parahaemolyticus-infected control shrimp.
The most likely explanation for this inconsistency between the two studies (in terms of survival rates) is associated with the different methods of experimental feed preparation, as mentioned above. The absence of histopathological lesions in the hepatopancreas and intestinal tissues of the IQ-fed shrimp but not the positive control shrimp also supports the protective effect of IQs.
The bioavailability of sanguinarine and other IQs from M. cordata in shrimp and other aquatic animals has yet to be studied. However, it was reported that they were very poorly absorbed from the gastrointestinal (GI) tract of various species following oral administration. This characteristic is desirable for the prevention of digestive tract infection but may not be suitable for systemic infection. Given that the GI tract is one of the major routes of Vibrio spp. infection in shrimp after immersion challenge, it is reasonable to assume that the oral administration of IQ extracts could prevent Vibrio spp. colonization in the shrimp’s GI tract, thereby suppressing the disease outbreak, which we observed in our present study.
Perspectives
Our data showed that all isoquinoline alkaloid-fed shrimp in Experiment 1 had significantly enhanced survival rates and immune parameters compared to the control group, even though the growth performances were similar across all groups. In Experiment 2, all IQ-fed groups showed better growth performance and survival rates compared to the positive control.
The two IQ formulations at the two sanguinarine concentrations (1 and 1.5 mg/kg of feed) supplemented into the shrimp diets showed equivalent efficacies in terms of improving growth performance, survival rate, immune response, and resistance to V. parahaemolyticus infection of Pacific white shrimp. Other than in the positive control group, no histopathological lesions in the hepatopancreas and the intestine were found.
In summary, our results demonstrated the benefits of using IQs from M. cordata as feed additives for improving the growth performance, survival rate, immune responses and resistance to vibriosis of Pacific white shrimp.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Pavarist Bussabong
Faculty of Fisheries, Department of Fishery Biology, Kasetsart University, Bangkok, Thailand
-
Tirawat Rairat, Ph.D.
Faculty of Fisheries, Department of Fishery Biology, Kasetsart University, Bangkok, Thailand
-
Niti Chuchird, Ph.D.
Corresponding author
Faculty of Fisheries, Department of Fishery Biology, Kasetsart University, Bangkok, Thailand -
Arunothai Keetanon
Faculty of Fisheries, Department of Fishery Biology, Kasetsart University, Bangkok, Thailand
-
Putsucha Phansawat
Faculty of Fisheries, Department of Fishery Biology, Kasetsart University, Bangkok, Thailand
-
Kanokwan Cherdkeattipol
Faculty of Fisheries, Department of Fishery Biology, Kasetsart University, Bangkok, Thailand
-
Phongchate Pichitkul, Ph.D.
Faculty of Fisheries, Department of Fishery Biology, Kasetsart University, Bangkok, Thailand
-
Waraporn Kraitavin
Phytobiotics (Thailand) Co., Ltd, Huaykwang, Bangkok, Thailand


