Infectious myonecrosis can cause significant losses
Infectious myonecrosis (IMN) was recognized at Brazilian shrimp farms in 2003 as a distinct disease of Pacific white shrimp (Litopenaeus vannamei). The viral agent infectious myonecrosis virus (IMNV) was subsequently named after the disease it causes.
Infection by IMNV at affected farms can result in mortalities of 40 to 60 percent and significant economic losses. Because IMNV behaves similarly to Taura syndrome virus (TSV) in the sense that disease caused by the virus usually results in survivors that are assumed to possess a degree of resistance to IMNV infection.
Hence, there have been efforts by the authors’ laboratory and others to develop a laboratory challenge method similar to that developed more than a decade ago for the selection of TSV-resistant lines of L. vannamei.
Early challenge development
To permit comparisons of different families for IMNV resistance, the development of a simple, standardized challenge protocol was necessary. Factors taken into account during development of the protocol included the requirement that the method produce over 50 percent mortality in an IMNV-susceptible reference line of specific pathogen-free (SPF) L. vannamei and that it be simple and repeatable. To accomplish this, it was necessary to first determine what exposure method would be reliable and produce consistent results.
An oral method was tested first, as it most closely mimics the natural route of infection in ponds – transmission through cannibalism. However, mortality was low, survival results were inconsistent and the prevalence of diagnosable IMNV infections in challenged shrimp was low, even several weeks after challenge.
Challenge by injection studies have resulted in higher mortality rates and higher prevalence of IMNV infections. Hence, this challenge route was explored for evaluating resistance among selected L. vannamei families.
Stress test
Early trials were run for 30 to 50 days with a stress test consisting of a sudden salinity drop performed six to seven days after the introduction of IMNV, when at least 50 percent percent of the population displayed opaque tail muscles indicative of IMNV infection. The stress tests did not result in significant mortality related to IMNV infection. The gross signs of IMN progressed with or without added stress, although the stress increased the prevalence of IMN signs within two days.
Eliminating the stress test from the IMNV challenge protocol provided a better challenge model because mortalities resulting directly from the sudden salinity drop occurred in both infected and non-challenged controls. Results from additional challenge studies also indicated that IMNV challenges can be run for only 20 days, with no significant differences in survival between 20 and up to 50 days post-challenge.
IMNV-resistant shrimp line
Part of the problem in developing a reliable IMNV challenge method has been the use of an apparently IMNV-resistant stock. In all studies at the University of Arizona, the reference stock used in initial work was the Kona line of SPF L. vannamei developed by the United States Marine Shrimp Farming Consortium.
Ironically, the Kona line demonstrates poor resistance to viral pathogens such as TSV and white spot syndrome virus. However, numerous IMNV challenges have resulted in 95 to 100 percent survival, with Kona shrimp sampled from these studies showing variable, but generally low-grade IMNV infections. Neither oral nor injection studies with this stock have resulted in significant mortality, although the shrimp consistently become infected with IMNV.
It was not until other lines of L. vannamei were provided to the university for challenge with TSV and IMNV that a comparison was possible among lines of selected industry stocks. When the stocks selected for TSV resistance and other features showed variable mortality rates that were often much lower than those found with Kona line controls in the same study, it became evident that the Kona line is resistant to IMN and/or IMNV infection. Furthermore, these unexpected findings indicated the utility of the injection method for challenge studies with IMNV.
Current challenge
The current IMNV challenge model design is very similar to the TSV challenge model developed at the University of Arizona (UAZ) in 2002. Briefly, for IMNV challenge, 1,000-L fiberglass tanks or 90-L glass aquariums were stocked in most studies at a maximum density of 0.5 grams/L, and all tanks contained their own biological filters. Each tank was kept covered to contain aerosols.
Challenge studies for IMN resistance run by UAZ have differed in the numbers of family lines assessed, with individual challenge studies including up to 13 family lines. The standard protocol for each challenge, regardless of tank size, consisted of a variable number of IMNV challenge tanks, at least one negative control tank containing representatives from all families challenged with IMNV and a positive control consisting of the Kona reference line. Each challenge study was run for a minimum of 20 days, when survival rates for each family line tested were determined.
The shrimp families utilized in these studies were provided by companies engaged in programs to select for TSV-resistant strains of L. vannamei. Most of the shrimp challenged at UAZ have ranged from 1.0 to 3.0 grams. When multiple family lines were presented for IMNV challenge, each family was tagged by the supplier prior to shipment with color-coded elastomer tags for ease in identification.
In most challenges, the Kona reference line is included as a positive control to ensure that the IMNV inoculum used for the challenge was infectious. Even though the Kona line does not suffer high mortalities from IMNV infection, the shrimp consistently become infected with IMNV and provide a reference point and confirmation that the inoculum is infectious.
Test method
In UAZ challenge studies, minced shrimp carcasses infected with the Brazilian isolate of IMNV were homogenized prior to clarification by centrifugation. The supernatant fluid was collected, dispensed into 1-ml tubes and frozen at minus-80 degrees-C until used.
At the start of the study, each shrimp was injected in the abdomen with approximately 20 µl of a clarified tissue homogenate diluted 1:5 with sterile 2 percent saline. Negative control shrimp were given the same volume of sterile 2 percent saline. All IMNV-exposed shrimp and negative control shrimp were fed a maintenance diet of commercial feed once daily.
Mortalities, survivors
Presentation of opaque, white tail muscle, indicative of IMNV infection, was noted in IMNV-challenged shrimp beginning on day 6 of the study. The first mortalities were noted on day 10, after which tanks were checked at least twice daily for the remainder of the study. All dead and moribund animals were removed, identified as to family line or cross and recorded.
Dead shrimp were frozen at minus-80 degrees-C and in some cases IMNV infection was confirmed and quantified by quantitative real-time polymerase chain reaction using both frozen shrimp collected dead during the study or as samples of survivors at termination. A few moribund animals and a sampling of survivors were also preserved in fixative to confirm by routine histology that IMNV infection was the cause of morbidity.
The family lines of surviving animals were identified and recorded at the termination of the study. Additionally, histological examination of five shrimp preserved in fixative prior to the start of the challenge documented the population’s IMNV-free health status upon arrival at the research facility.
Results
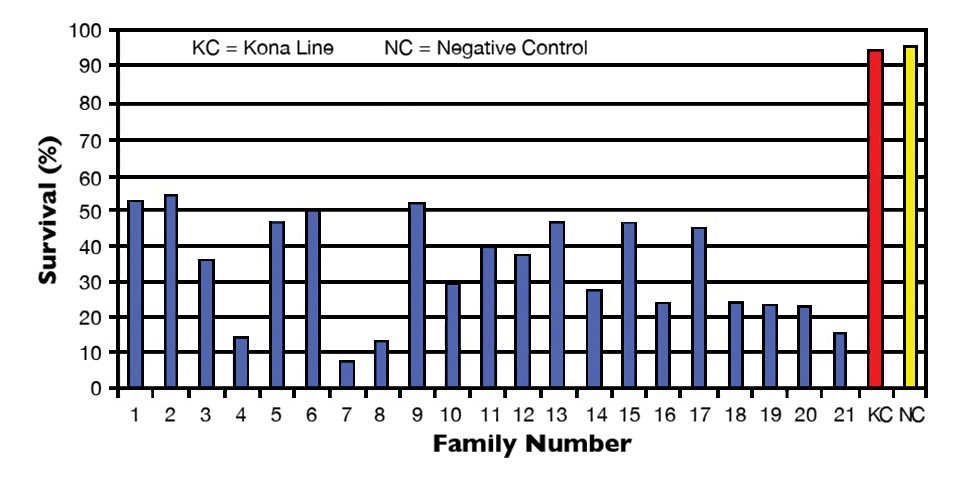
The results discussed here were compiled from three separate challenges performed on L. vannamei from three Hawaii-based shrimp-breeding companies. A total of 21 L. vannamei family lines were challenged with IMNV.
Overall, survival ranged 7 to 54 percent in all family lines tested in 2009. Mean survival of the Kona line positive controls was 95 percent, while mean survival of the negative controls was 96 percent. In all studies, IMNV infection was confirmed in the challenged shrimp.
Although still being refined, the IMNV challenge method described has shown promise as a useful tool to measure IMN resistance in selected family lines of L. vannamei. Families that show high survival can potentially be used as broodstock to produce postlarvae for stocking into areas where IMNV is enzootic, as was previously done to produce TSV-resistant stocks for regions where TSV is enzootic.
(Editor’s Note: This article was originally published in the July/August 2010 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-

Brenda L. White-Noble
Department of Veterinary Sciences & Microbiology
University of Arizona
1117 East Lowell
Tucson, Arizona 85721 USA[117,100,101,46,97,110,111,122,105,114,97,46,103,97,64,101,116,105,104,119,98]
-

Donald V. Lightner, Ph.D.
Department of Veterinary Sciences & Microbiology
University of Arizona
1117 East Lowell
Tucson, Arizona 85721 USA -

Kathy F.J. Tang, Ph.D.
Department of Veterinary Sciences & Microbiology
University of Arizona
1117 East Lowell
Tucson, Arizona 85721 USA -
Rita Redman, H.T.
Department of Veterinary Sciences & Microbiology
University of Arizona
1117 East Lowell
Tucson, Arizona 85721 USA
Tagged With
Related Posts

Health & Welfare
Brazil shrimp farm performs genetic selection for IMNV resistance, growth
The Queiroz Galvão Alimentos shrimp farm and hatchery in Brazil have been working with Concepto Azul to implement a disease-prevention and genetic-breeding program that addresses ongoing impacts from infectious myonecrosis virus (IMNV) and other pathogens.

Health & Welfare
A comprehensive look at the Proficiency Test for farmed shrimp
The University of Arizona Aquaculture Pathology Laboratory has carried out the Proficiency Test (PT) since 2005, with 300-plus diagnostic laboratories participating while improving their capabilities in the diagnosis of several shrimp pathogens.

Responsibility
A look at various intensive shrimp farming systems in Asia
The impact of diseases led some Asian shrimp farming countries to develop biofloc and recirculation aquaculture system (RAS) production technologies. Treating incoming water for culture operations and wastewater treatment are biosecurity measures for disease prevention and control.

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 1
Indian shrimp hatcheries have experienced larval mortality in the zoea-2 stage, with molt deterioration and resulting in heavy mortality. Authors investigated the problem holistically.


