Species requires a high-quality live prey of proper nutritional value

The yellowtail snapper (Ocyurus chrysurus) is one of several snapper species that are abundant in the Bahamas, off south Florida, USA, and in the Caribbean. Unlike other snapper species, the fish easily reproduce under controlled conditions and will spawn “naturally” on a year-round basis. This makes yellowtails a good aquaculture candidate and excellent model species for the development of larvae culture techniques for snappers.
As with other snappers, yellowtails produce very small larvae that are a challenge to rear. Initial protocols for larval rearing have resulted in overall survival of about 3 percent from egg to advanced juvenile. Results have been unpredictable, presumably due to nutritional inadequacies and/or variability of the larval diets.
Larval-rearing protocols
The maturation and larval rearing of yellowtail snappers have been reported by several authors. The basic protocols the authors of this article found to work best include a mixture of clear and green water culture techniques.
Larvae culture is best initiated with the introduction of fertilized eggs into presterilized clear water. Two days after hatching, green water techniques are initiated with the addition of Nannochloropsis oculata algae and Brachionus plicatilis rotifers to the culture water. For days 2 to 7, the algae is maintained in quantities sufficient to produce a light green tint to the culture water (approximately 1 x 105 – 2 x 105 cells per millileter) and provide sufficient nutrients for growth and reproduction of the rotifers.
Starting at day 7, the system is converted back to clear water management, with the culture water recirculated in the evenings through biological and particulate filters. Enriched rotifers are added twice daily from days 7 to 18 such that 5 to 10 rotifers per milliliter are maintained in the culture water.
Newly hatched artemia nauplii are added days 14 to 16, followed by 2-day-old enriched artemia nauplii for the remainder of the production period. Dry feed is also added on day 7 and continue throughout production.
Culture system and eggs

The larval-rearing trials were conducted in a series of 1,000-liter tanks, each containing a center drain, two air stones, and water source. Water level was controlled by an internal stand pipe surrounded by an external pipe screened with 48- or 250-μ mesh netting. Effluent water from each tank ran through a common drain line and 100-μ and 5-μ felt filter bags, followed by a biological filter. Filtered water was then pumped back into the culture tanks or discharged during water exchanges.
Eggs were collected from first-generation laboratory-reared broodstock maintained using protocols similar to those described by Turano et al. in the Journal of the World Aquaculture Society in 2000. The collected eggs were held in a solution of 1 percent formalin for one hour, then transferred into the culture tanks.
Evaluation of enrichment protocols
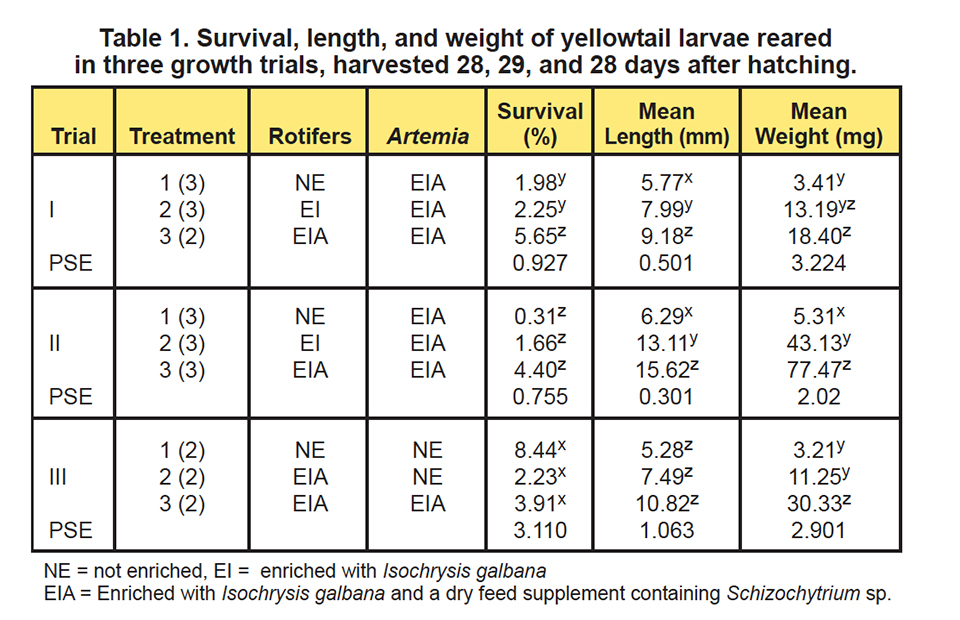
To optimize the nutritional quality of the live prey used to feed the larval fish, three larval-rearing trials were conducted to evaluate the need for rotifer and artemia enrichment. In the first growth trial, each tank was stocked with 6,000 yellowtail eggs at the tail-bud stage, whereas in the remaining trials, 4,000 eggs were stocked.
During the first and second trials, three live feed enrichment protocols were evaluated: Treatment 1, mass-produced rotifers reared on Nannochloropsis oculata and yeast; Treatment 2, rotifers enriched overnight with Isochrysis galbana; and Treatment 3, rotifers enriched overnight with Isochrysis galbana and a dry feed supplement containing Schizochytrium sp.
All rotifers were harvested in the morning and either directly fed or held for enrichment. The rotifers and artemia were thoroughly rinsed with filtered seawater immediately prior to feeding.
A third growth trial, which evaluated the need for enrichment of artemia, included the following treatments: Treatment 1, nonenriched rotifers and enriched artemia; Treatment 2, rotifers enriched with Isochrysis galbana and a dry feed supplement containing Schizochytrium sp., and non-enriched artemia; and Treatment 3, rotifers and Artemia enriched with Isochrysis galbana and the Schizochytrium supplement.
At the conclusion of each production trial, survival, final weight, and lengths of the fish were determined (Table 1). Additionally, subsamples of the live food items and fish were collected and analyzed for their fatty acid contents.
Results
Live foods are often good sources of nutrition. However, for many species of fish larvae, these traditional food sources have been found inadequate to support larval development. Even in instances where these prey items are considered sufficient, the nutritional quality of live prey can vary considerably, resulting in increased variability in larval-rearing success. Quite often this is due to a deficiency of essential fatty acids, which results in fin rot, shock syndrome, improper development of the swim bladder, inflammation of the heart muscle, reduced growth rate and increased mortality.
Enhancing PUFA files
Essential fatty acids are part of a general group of lipids know as poly-unsaturated fatty acids (PUFA), which are common in marine oils. The use of enrichments to enhance the PUFA profiles of live foods is well established, and there are a number of products that work to stabilize or enhance the nutrient profiles of live foods. In general, these products are designed to enhance the levels of arachidonic acid, eicosapentaenoic acid, or doco-sahex-aenoic acid, singly or in combination.
Because “we are what we eat,” the fatty acid profiles of the live food generally reflect the food (and its enrichment products) on which it is grown. This “enrichment” occurs through the incorporation of lipids into the tissues of the live food and/or the loading of the enrichment into the gut of the live food. Consequently, to deliver the enriched nutrients to the larvae, the live food must be consumed relatively quickly. If prey items remain in the culture system, their nutritional value decreases. Because the use of enriched foods generally requires more complicated procedures and technical expertise, their use is often discouraged by hatchery personnel.
Unenriched rotifers nutritionally inadequate
The results of the first two larval-rearing trials (Figs. 1 and 2) showed an incremental increase in survival, length, and weight of the larvae. The poorest response was observed in fish offered live foods using typical mass-production techniques.
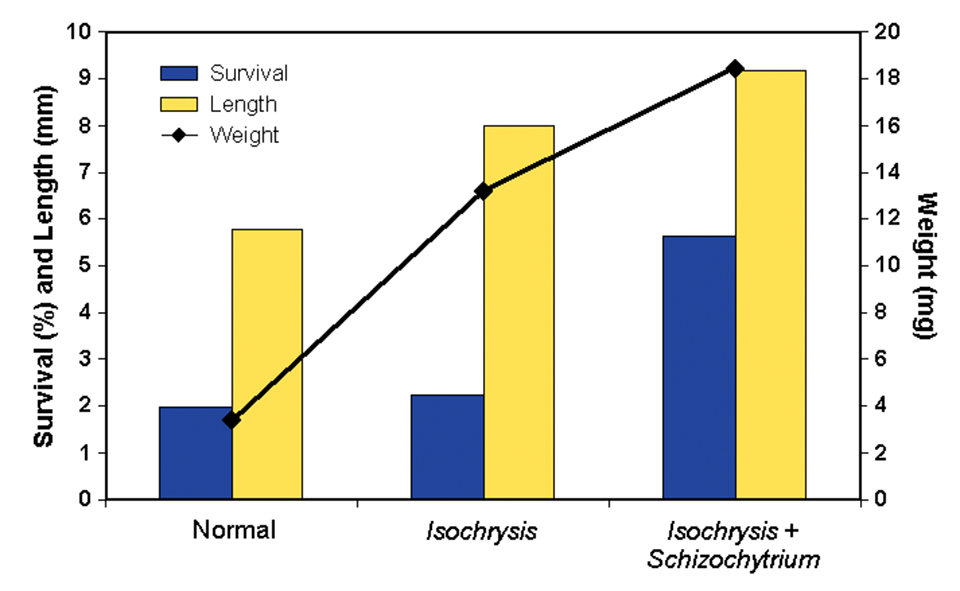
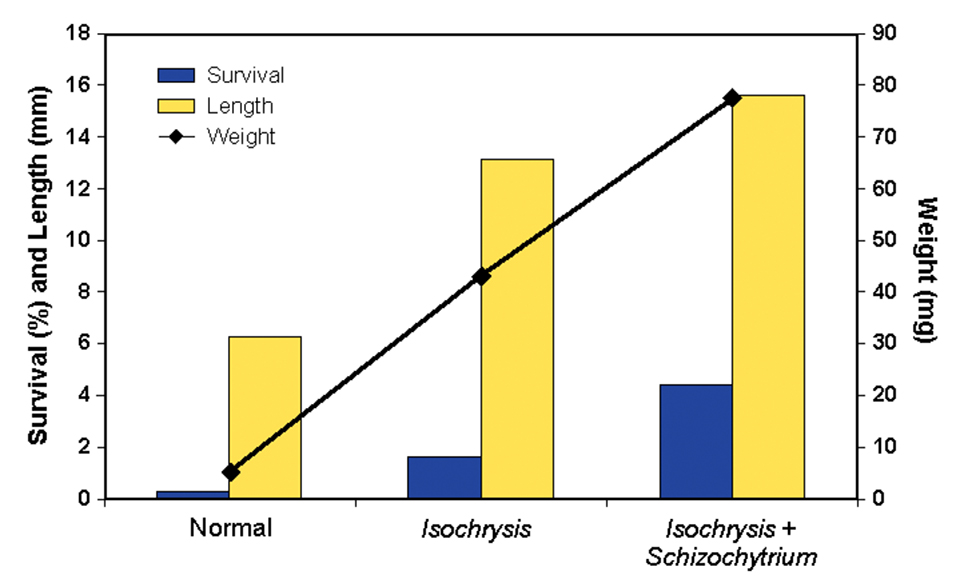
The holding of the rotifers for 24 hours in water with Isochrysis galbana yielded improved larval growth responses, but they were not as good as the growth observed when both Isochrysis galbana and the dry supplement containing Schizochytrium sp., were used. These results confirmed that unenriched rotifers are not nutritionally adequate for this species.
Artemia enrichment
The second component of this research evaluated the necessity for enrichment of the artemia. Results are presented in Fig. 3. Comparing the response of the larvae offered enriched rotifers and either nonenriched or enriched artemia, there were clear increases in the length, weight, and survival of larvae offered enriched artemia.
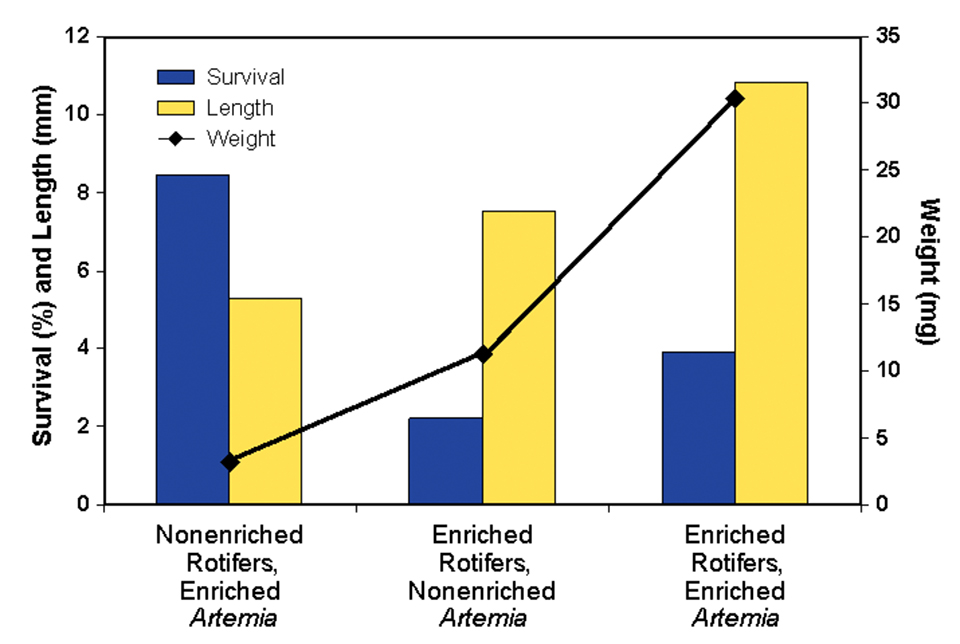
It is interesting to note that the high survival of larvae fed nonenriched rotifers and enriched artemia was essentially an artifact, as the larvae were held back in their development. If the study had been carried out for a longer period of time, these larvae would not have survived. The inadequacy of essential fatty acids during early development often inhibits development at later stages and cannot be mediated by later supplementation.
Conclusion
Yellowtail snapper larvae require a very high-quality live prey of proper nutritional value for the larvae to properly grow and develop. Based on communications with people working with mutton and red snappers, a similar response would be expected from these species.
Although not all species are as sensitive to variation in the nutritional quality of live food items, the use of enrichment protocols is clearly warranted for a number of species. Although the use of enrichments complicates the larval-rearing process, these procedures should be incorporated into most larval-rearing protocols for snapper species.
(Editor’s Note: This article was originally published in the April 2003 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
D. Allen Davis, Ph.D.
Department of Fisheries and Allied Aquacultures
Auburn University
204 Swingle Hall
Auburn, Alabama 36830 USA -
Deborah R. Smith
Department of Fisheries and Allied Aquacultures
Auburn University
204 Swingle Hall
Auburn, Alabama 36830 USA -
G.J. Holt, Ph.D.
University of Texas at Austin
Marine Science Institute
Port Aransas, Texas, USA
Tagged With
Related Posts

Intelligence
Bahamas venture focuses on grouper, other high-value marine fish
A new venture under development in the Bahamas will capitalize on Tropic Seafood’s established logistics and infrastructure to diversify its operations from processing and selling wild fisheries products to include the culture of grouper and other marine fish.

Health & Welfare
Tropical, subtropical marine fish hatchery technology needs improvement
Hatchery technology for most commercially important species of cultured marine fishes is essentially unchanged for the past several decades.

Aquafeeds
Research delves into enrichment of live feed for larval fish
A two-year grant of $276,000 seeks to improve the nutrition of live feed for, and therefore the production of, larval California yellowtail and halibut, with the hopes that the technology will be applicable to other species.

Intelligence
Sole show culture potential in Europe
Early efforts at sole culture in Europe were hampered by several technological and disease problems. Improvements in recirculation technology and advances in feed technology have renewed interest in the species.


