Novel procedure can detect Acute Hepatopancreatic Necrosis Disease in penaeid shrimp

Acute hepatopancreatic necrosis disease (AHPND) has caused substantial mortality in penaeid shrimp cultured in various countries in Southeast Asia and Latin America. The disease causes sloughing and necrosis of hepatopancreas tissue that results from toxins (pirA&Bvp) produced by specific strains of Vibrio parahaemolyticus.
Diagnosis of AHPND usually involves sacrificing the shrimp to collect hepatopancreas tissue for PCR analysis and/or histopathology. However, this invasive method is obviously not desirable for monitoring valuable broodstock, and non-sacrificial methods would be preferred. Non-invasive methods involving fecal testing have been successfully used for monitoring other enteric pathogens of shrimp.
As AHPND-bacteria are present in the digestive systems of infected shrimp, we based our research on the possibility that fecal samples could be collected for monitoring shrimp populations without causing mortalities. Here we report on our study (summarized from the original publication in Aquaculture Reports 5 (2017 58–61), where we evaluated procedures with shrimp fecal samples directly or with the enrichment of the bacteria present in the feces through culturing them in media. We thank Dr. Donald Lightner (School of Animal and Comparative Biomedical Sciences, University of Arizona, Tucson, AZ, USA) for his assistance during this study.
The use of feces as diagnostic samples from asymptomatic shrimp
This trial was designed to determine if fecal samples from AHPND-affected large juvenile shrimp can be used as diagnostic specimen. Fig. 1 (below) depicts the procedures we developed during our study. Pacific white shrimp, Penaeus vannamei, (four SPF shrimp, average weight of 8.5 grams) were fed once with shrimp pellets soaked in the AHPND-V. parahaemolyticus culture (108 CFU/mL, a sub-lethal dosage). After feeding, the shrimp were rinsed with formalin-iodine to disinfect the residual AHPND-bacteria during the per os exposure, and were then transferred into four individual tanks.
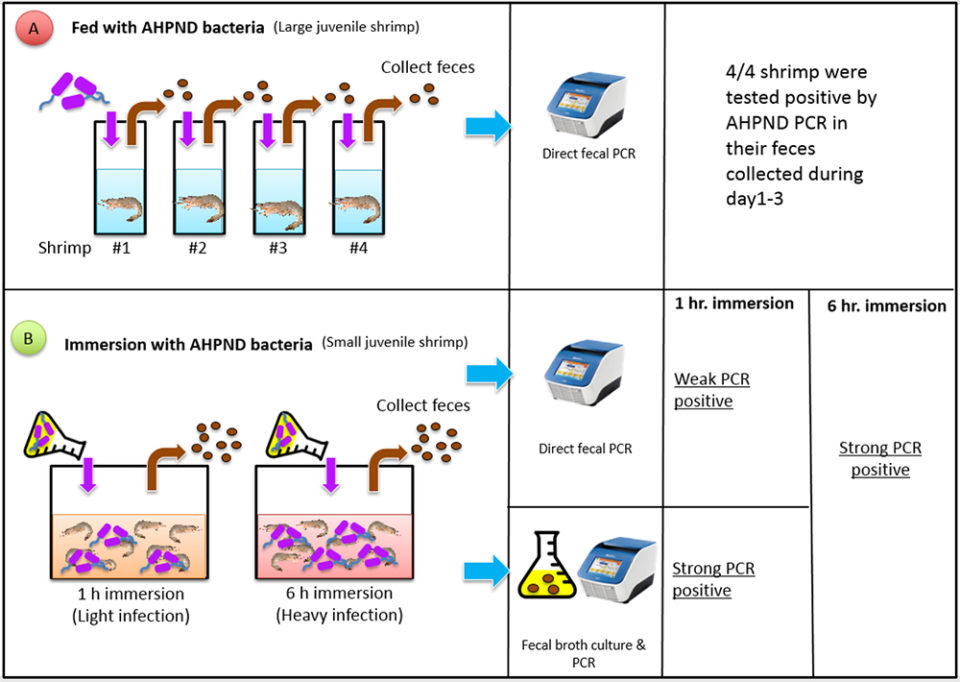
Fecal strands were collected during the three-day experimental period; then DNA was extracted and followed by an AHPND PCR targeting both toxin genes, pirAvp and pirBvp. The results showed strong positive bands after the agarose gel electrophoresis. There was no mortality in these shrimp during the trail. Upon termination, the shrimp were processed for histopathological examinations and no AHPND lesions were detected. This indicates that feces can be used as diagnostic samples for monitoring large shrimp that have asymptomatic, low levels of infection.
PCR sensitivity comparisons of templates prepared from fecal DNA or from enriched bacterial culture
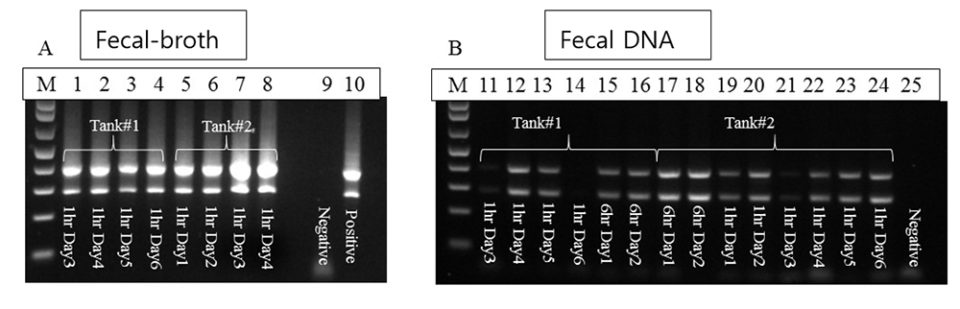
SPF P. vannamei (80 shrimp, average weight: 0.7 g) were used in two immersion bioassays, 1- and 6-hour immersion with AHPND-V. parahaemolyticus broth. The fecal samples were collected after 24 hours from each bioassay. One part of the fecal samples was extracted for DNA and used directly for PCR analyses, and one part of the feces samples was cultured with TSB+ media (at a ratio of 1:1000) and incubated at 28 to 29 degrees-C for 6 hours; this broth was then used as a PCR template without DNA extraction.
With the 1-hour immersion, shrimp became moribund at day 4, with a cumulative mortality of 45 percent at termination (day 6). Twelve fecal samples were sampled and analyzed, by PCR: seven samples from the extracted DNAs had strong positive PCR results, four were weak positive and one sample was not detected. With the 6-hour immersion, shrimp became moribund at day 1 and all the shrimp died by day 2. Strong PCR amplifications were observed in both fecal DNA extract and enriched bacterial culture.
From both bioassays, we found that the enrichment method had stronger PCR band intensities than fecal DNA samples (Fig. 2), as the enrichment greatly increased the bacterial populations. The increased sensitivity from enrichment method can circumvent the use of nested PCR for AHPND detection.
Perspectives
Our study showed that not all infected shrimp succumb to the AHPND disease. Likewise, in shrimp farms, animals infected with sub-lethal doses might recover from disease and become asymptomatic carriers. The diagnosis, screening, and monitoring of AHPND typically involves sacrificing individuals to obtain hepatopancreas tissue samples, a procedure that is undesirable for use with high-value broodstock.
The diagnostic test we developed and describe here for AHPND does not require sacrificing shrimp, especially for those asymptomatic survivors of valuable broodstock populations. The test involves PCR analysis of an enriched broth culture of bacteria from fecal samples. An enriched broth can be used to detect AHPND-V. parahaemolyticus in feces from both moribund and asymptomatic animals.
These findings are of interest to shrimp producers regarding the development of strategies for disease management of this serious disease AHPND, and will prove very useful in the diagnosis and monitoring of AHPND in farmed shrimp populations.
Authors
-

Jee Eun Han, D.V.M. Ph.D.
*Corresponding author
CJ CheilJedang Feed & Livestock Research Institute, Korea
School of Animal and Comparative Biomedical Sciences
University of Arizona
-
Patharapol Piamsomboon, D.V.M. Ph.D.
Veterinary Science
Prince of Songkhla University
Songkhla, Thailand
-

Kathy F.J. Tang, Ph.D.
School of Animal and Comparative Biomedical Sciences
University of Arizona
Tucson, AZ 85721 USA
Tagged With
Related Posts

Health & Welfare
Four AHPND strains identified on Latin American shrimp farms
Two virulence genes are known to encode a binary photorhabdus insect-related toxin that causes acute hepatopancreatic necrosis disease in shrimp. The pathogenicities of these V. campbellii strains were evaluated through laboratory infection and subsequent histological examination in P. vannamei shrimp.

Health & Welfare
Genetic variation for resistance to WSS, AHPND in Pacific white shrimp
Selection for disease resistance has been used in breeding farm animals and can be a viable option to deal with white spot syndrome and acute hepatopancreatic necrosis disease in commercial shrimp culture. In trials, heritability for AHPND resistance was low, while that for WSS was moderate.

Health & Welfare
PCR methods characterize AHPND V.p isolates
In analyzing the plasmid sequence from the whole genome sequences of AHPND V. parahaemolyticus (V.p) isolates, researchers identified a clear geographical variation within the plasmid, and developed PCR methods to characterize AHPND V.p isolates as either Mexico-type or Southeast Asia-type.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.


