Genome-wide association study identifies genetic variants

Atlantic salmon (Salmo salar) is an important farmed fish species, known for its high content of the health-promoting omega-3 long-chain polyunsaturated fatty acids (omega-3 LC PUFA) eicosapentaenoic (EPA; 20:5 omega-3) and docosahexaenoic acid (DHA; 22:6 omega-3) in muscle. Levels of omega-3 LC PUFA have decreased significantly the last few decades because of the substitution of a large portion of marine ingredients with more sustainable plant ingredients in the diet of farmed Atlantic salmon. Quantitative genetic analyses have estimated genetic parameters of Atlantic salmon omega-3 fatty acids (FAs) and showed the potential of selective breeding to increase omega-3 LC PUFA levels in salmon fillets, as they are heritable traits.
Several studies have observed a higher selective deposition of DHA in muscle when compared to other dietary fatty acids. EPA, on the other hand, seems to be metabolized to a larger extent than DHA in muscle. A recent study of Atlantic salmon by our group found that muscle EPA content was negatively associated with expression of genes involved in lipid catabolism and hypothesized that individual differences in EPA content is caused by selective oxidation of fatty acids. There is, however, limited knowledge on which genes and metabolic pathways that are most important for determining EPA and DHA content in Atlantic salmon muscle.
Genome-wide association studies (GWAS) are a powerful tool that can identify Single Nucleotide Polymorphisms [SNPs – a substitution of a single nucleotide (the basic building blocks of DNA and RNA) that occurs at a specific position in the genome (complete set of DNA of any organism)] linked to genes associated with phenotypic (observable) traits. GWAS of muscle FA composition have been performed in cattle and pigs, where several significantly associated regions and loci have been identified. In fish, one study in Asian sea bass (or barramundi, Lates calcarifer) showed the complexity of the genetic architecture of FAs in fish.
To increase the understanding of the genetics of muscle omega-3 LC PUFA content in Atlantic salmon, GWAS is a relevant approach that has not yet been explored in Atlantic salmon. This article – adapted and summarized from the original publication [Horn S.S. et al. 2020. GWAS identifies genetic variants associated with omega-3 fatty acid composition of Atlantic salmon fillets.] – reports on a study to identify genetic variants associated with long-chain omega-3 fatty acid content in Atlantic salmon muscle, by performing a genome-wide association study (GWAS).
https://www.aquaculturealliance.org/advocate/omega-3s-levels-fall-in-farmed-salmon-but-its-still-a-top-source/
Study setup
A total of 642 fish from 92 sires and 194 dams were included in the analysis. All sires had four or more offspring from more than one dam. The fish originated from one year-class of the Atlantic salmon breeding population of SalmoBreed AS. The fish were transferred to sea at a mean weight of 0.1 kg, and slaughtered approximately 12 months later, at a mean weight of 3.6 kg (ranging from 1.2 to 6.4 kg). The fish were fed a commercial broodstock feed from Skretting containing 70 percent fish oil and 3.1 percent each of EPA and DHA.
All the fish were reared under the same conditions and were fasted 13 to 14 days prior to slaughter. After sacrifice, sex was determined visually by inspection of the gonads, body weight was recorded and skeletal muscle samples for lipid and FA analysis were taken from Norwegian Quality Cut, collected, frozen and stored at minus-20 degrees-C for various analyses. For detailed information on fish population and recordings, muscle fat and fatty acid analysis, genotyping and statistical analyses, refer to the original publication. This work was supported by the Norwegian research council (Grant number NFR 244200).
Results and discussion
In this study, GWAS was performed on 642 slaughter sized Atlantic salmon displaying variation in EPA and DHA content. Several traits were analyzed, and most traits did not show genome-wide significant probability (p-values) values. The exceptions were DHA/ALA [α-linolenic acid] ratio and DHA/DPA [docosapentaenoic acid] ratio. Therefore, our focus was on these two traits, in addition to the major omega-3 LC PUFAs: DHA and EPA.
Table 1 shows descriptive statistics for these traits. The mean content of EPA and DHA was 5.42 and 6.75 percent of total muscle FA, respectively. EPA content displayed much greater variation and lower heritability [a statistic used in breeding and genetics to estimates the degree of variation in a phenotypic trait in a population that is due to genetic variation between individuals in that population] compared to DHA. The ratio-traits had slightly higher genomic heritability-estimates than DHA, with 0.21 for DHA/DPA and 0.28 for DHA/ALA.
Horn, omega-3, Table 1
| Trait | Mean (SD) | Min | Max | CV | h^2 (SE) |
|---|
Trait | Mean (SD) | Min | Max | CV | h^2 (SE) |
|---|---|---|---|---|---|
| Fatty acids^* | |||||
| EPA 20:5n-3 | 5.29 (1.21) | 2.06 | 9.45 | 22.94 | 0.05 (0.04) |
| DHA 22:6n-3 | 6.51 (1.16) | 1.4 | 9.1 | 17.87 | 0.23 (0.07) |
| Ratios | |||||
| Ratio DHA/ALA | 1.94 (0.27) | 0.91 | 3.1 | 13.95 | 0.28 (0.07) |
Ratio DHA/DPA | 2.87 (0.30) | 1.94 | 4.25 | 10.57 | 0.21 (0.06) |
^*Percentage of total FA in muscle. SD: standard deviation; Min: minimum value; Max: maximum value; CV: coefficient of variation (SD/mean x 100); h^2: genomic heritability; SE: standard error.
We found a candidate gene for DHA/DPA ratio directly involved in omega-3 bioconversion activity. However, we did not find a signal in or near genes known to be involved in omega-3 bioconversion for the traits EPA, DHA or DHA/ALA ratio. The genes of this pathway should supposedly have been identified in these analyses if this pathway was important for determining EPA and DHA content in muscle. Therefore, our results suggest that the genes of the omega-3 bioconversion pathway are not clearly associated with EPA and DHA content in muscle of Atlantic salmon.
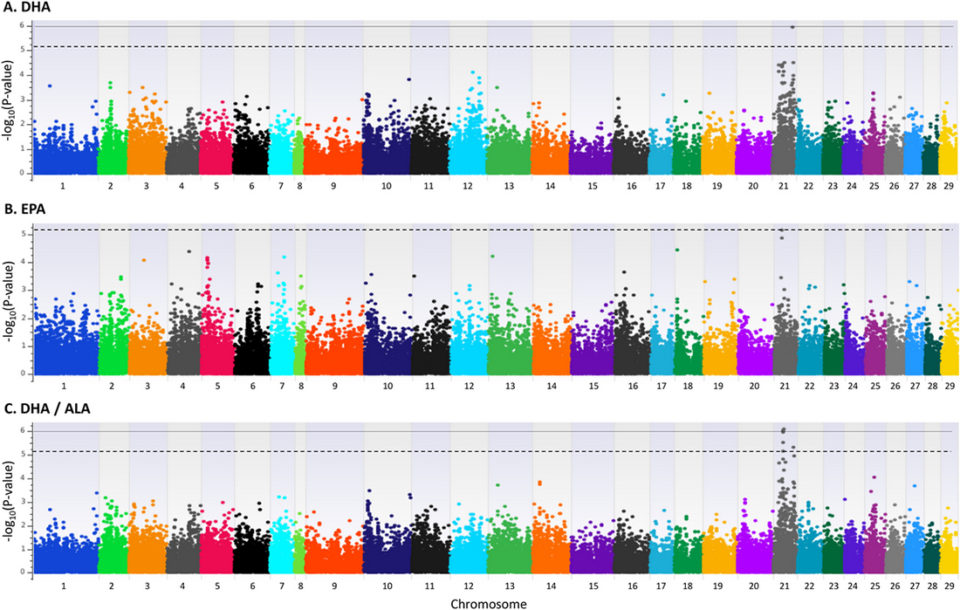
A potential major factor behind the lack of association with omega-3 bioconversion pathway genes is that the fish was fed a high fish oil diet. High dietary levels of EPA and DHA suppress the activity of the omega-3 bioconversion pathway. The starvation period may also have affected the metabolic activity and fatty acid composition. The differences observed in omega-3 content are therefore more likely caused by differences in other metabolic processes rather than omega-3 bioconversion.
Another possible explanation is that although omega-3 bioconversion is important for fatty acid composition in the liver, it might not be as important for muscle fatty acid composition. The role of this pathway in determining muscle fatty acid composition has not been shown, and long-chain omega-3 fatty acid content in liver and muscle appear to be two different traits, as they display a very low correlation.
Our results suggest that genetic variation affecting fillet EPA and DHA content is present on Atlantic salmon chromosome 21. The GWAS results for DHA to ALA ratio further strengthened the significance of chromosome 21 in relation to omega-3 LC PUFA content in Atlantic salmon muscle. The region of the most significant SNPs on chromosome 21 contained several uncharacterized genes. Some of these might prove to be involved in omega-3 FA metabolism in the future. Additional studies – including fine mapping and functional validation – are needed for further clarification of the variants underlying the FA composition traits.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Siri S. Horn
Ph.D. candidate and corresponding author
Nofima, Norwegian Institute of Food, Fisheries and Aquaculture Research
P.O. Box 210, N-1432 Ås, Norway; and
Department of Animal and Aquaculture Sciences
Norwegian University of Life Sciences
N-1430 Ås, Norway -
Bente Ruyter, Ph.D.
Nofima, Norwegian Institute of Food, Fisheries and Aquaculture Research
P.O. Box 210, N-1432 Ås, Norway -
Prof. Theo H.E. Meuwissen
Department of Animal and Aquaculture Sciences
Norwegian University of Life Sciences
N-1430 Ås, Norway -
Hooman Moghadam, Ph.D.
SalmoBreed AS
Sandviksboder 3A, N-5035 Bergen, Norway -
Borghild Hillestad, Ph.D.
SalmoBreed AS
Sandviksboder 3A, N-5035 Bergen, Norway -
Anna K. Sonesson, Ph.D.
Nofima, Norwegian Institute of Food, Fisheries and Aquaculture Research
P.O. Box 210, N-1432 Ås, Norway
Tagged With
Related Posts

Intelligence
Are omega-3s in farmed jade perch as high as believed?
Farmed jade perch – similar to other cultured fish species – is only rich in omega-3 fatty acids if its diet consists of these nutrients in high amounts. How does it compare to the wild fish?

Aquafeeds
Bridging the omega-3 gap with methane, microalgae
Innovation is leading to new ingredient options for renewable sources of omega-3 fatty acids. But Replicating long chain fatty acids is a tall order, Advocate contributor Lisa Duchene discovered.

Intelligence
Wanted: More omega-3s. But from where?
Cargill and Australia’s Nuseed are both investing large sums of money in the development of a genetically modified canola oil rich in DHA. Meanwhile, a leading nutritionist casts doubts on the necessity of omega-3s from fish.

Aquafeeds
Veramaris runs away with F3 oil-alternative contest
The DSM-Evonik joint venture won the F3 Fish Oil Challenge by a wide margin. CEO Karim Kurmaly talks about the “sacrifice” his team made to win.


