The emerging freshwater species’ nutritional profile is highly dependent on its diet

The jade perch (Scortum barcoo) is a freshwater omnivorous fish with a heavily built body paired with a small head. The species is also distinguished by the presence of one or more black spots on its body (Fig. 1). Native to Australia, the species is being farmed in recirculating systems and intensive ponds in China, Malaysia and Singapore. This is largely due to its relatively fast growth rate and acceptance of pelleted feeds in aquaculture conditions.
Much of the market interest in this species is due to its appealing taste and reportedly high omega-3 fatty acid content. Omega-3 fatty acids can refer to α-linolenic acid (18:3n-3), which can be found in high amounts in some plant oil sources such as linseed, or those with longer chains such as EPA (20:5n-3) and DHA (22:5n-3) that are more often associated with wild-caught marine fish. Omega-3 fatty acids are well known to be healthful for the human consumer over fatty acids that are saturated (SFA), monounsaturated (MUFA) or omega-6, which has promoted the jade perch’s healthy image.
The origin of this image is based on fish collected from the wild. These fish would have been feeding on natural foods containing significant amounts of long-chain omega-3 fatty acids, such as EPA and DHA. Nevertheless, the fish was subsequently advertised online as being a healthier and more attractive alternative to other farmed freshwater fish, such as tilapia, which in the past has erroneously received negative publicity as having a similar nutritive value as bacon.
This is not wholly without merit and is a reflection of the diets provided to farmed fish. However, there are indications that Jade perch is no different. This is because, in a later study when jade perch were fed fish oil- or vegetable oil-based diets, the levels of longer chain omega-3 fatty acids were substantially less for fish fed the vegetable oil-based diets. Since the fatty acid composition of the diets was not directly measured, it is unclear whether Jade perch can accumulate omega-3 fatty acids at levels higher than their diets.
Determining this information should be of interest for farmers and consumers of jade perch, as this industry is expected to increase in production and spread to other countries. Therefore, the objective of our study was to feed the species a commercial pellet for two months, with a relatively high omega-3 content, and compare the fatty acid composition between the fish and diets.
The proximate composition of jade perch was also measured and compared with those of other commercially important fish. To minimize any chances of obtaining natural feeds that could potentially influence the results, jade perch were cultured in a closed recirculating system.
Feeding trial and fatty acid measurements
Jade perch fingerlings (Fig. 2) were purchased from SA Agromax Enterprises, Petaling Jaya, Selangor, Malaysia, and transferred to Sepang Today Aquaculture Centre (STAC), Pelek, Selangor. During their acclimation, the fish were fed commercial floating pellets (Star Feedmills, Malaysia; crude protein of 43 percent and crude lipid of 6 percent), designed for Asian sea bass (Lates calcarifer) three times each day. This feeding frequency was chosen based on our prior research showing this was optimal for growth (Al-Khafaji et al. 2017), while these pellets were chosen based on their relatively high omega-3 content.

The fish (initial weight of 7.3 grams) were equally stocked into three fiberglass tanks (1 cubic meter, Fig. 3) containing 10 fish each and fed the same commercial pellet. Each tank received gentle aeration and all tanks were attached to a recirculating system, equipped with a mechanical and biological filter. Tap water was used in the tanks and lime was applied to maintain a pH of around 7.8. A partial water exchange of 20 percent was performed each week.

After two months of feeding the fish three times daily to satiation, they had an approximate weight gain and survival of 700 percent and 96 percent, respectively. The fish whole-body and muscle were analyzed for their proximate composition according to standard research procedures. For the fatty acid composition, both the diets and muscle of jade perch were measured by first extracting the lipids using a chloroform and methanol (2:1 ratio). The fatty acids were then transmethylated to their fatty acid methyl esters (FAME) and measured for their fatty acid composition according to Ebrahimi et al. (2014).
Nutritive value
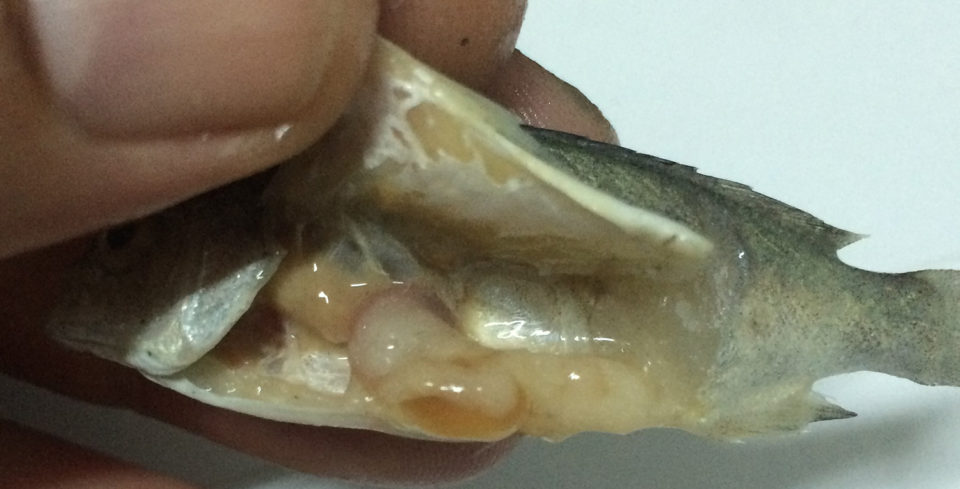
The whole-body and muscle crude protein levels of jade perch are comparable with those of other fish species, such as tilapia and catfish. However, the lipid content was substantially higher, particularly in the whole body (Table 1). This may be due to the relatively high water temperatures of 27 degrees-C and consumption upwards of 10 percent of their body weight, likely leading to the accumulation of internal lipid stores (Fig. 4).
Therefore, when comparing the muscle lipid content of jade perch under these conditions with other fish species (Table 2), it could be considered as an oily fish. Oily fish such as salmon and trout, however, tend to be high in longer chain omega-3 fatty acids, whether they are farmed or caught from the wild (Blanchet et al. 2005).
Romano, jade perch, Table 1
| Moisture | Crude protein | Crude lipid | Crude ash | |
|---|---|---|---|---|
| Whole body | 66.2 | 15.4 | 12.8 | 3.4 |
| Muscle | 63.8 | 18.7 | 7.2 | 0.8 |
Romano, jade perch, Table 2
| Species | Moisture | Crude protein | Crude lipid | |
|---|---|---|---|---|
| Carp 1 | Cyprinus carpio | 78 - 80 | 17.5 - 18.9 | 2.0 - 2.2 |
| Farmed tilapia 2 | Oreochromis sp. | 76 - 78 | 18.5 - 19.0 | 1.6 - 1.8 |
| Wild tilapia 3 | Oreochromis niloticus | 81.3 | 13.6 | 0.54 |
| Catfish 1 | Anarhichas sp. | 78 | 17.0 - 19.7 | 2.1 - 3.8 |
| Eel 1 | Anguilla anguilla | 60 - 71 | 14.4 | 8.0 - 31.0 |
| Perch 1 | Perca fluviatilis | 79 - 80 | 17.6 - 19.0 | 0.8 |
| Salmon 1 | Salmo salar | 67 - 77 | 21.5 | 0.3 - 14.0 |
1 data compiled from http://www.fao.org/wairdocs/tan/x5916e/x5916e01.htm
2 data from Ng et al. (2013)
3 data from Olopade et al. (2016)
In contrast, both the SFA and MUFA content in jade perch were substantially higher than their omega-3 content. It is well known that the fatty acid composition of fish is largely dependent on, and generally similar, to those of the diet. This is certainly the case with jade perch.
But what makes these results more remarkable is that both the SFA and MUFA content were higher in jade perch compared to their diets, while the omega-3 content was lower. These results clearly indicate that the jade perch is not outstanding in terms of its omega-3 content. This is particularly valid when considering that other freshwater fish cultured in tropical conditions, such as tilapia, had a much higher content of DHA (C22:6n-3), regardless of being fed a fish oil or vegetable oil based diet (Table 4).
Romano, jade perch, Table 3
| Fatty acid | % Diet | % Muscle |
|---|---|---|
| C12 | 0.00 | 0.12 |
| C14 | 3.45 | 3.74 |
| C15 | 0.18 | 0.00 |
| C15:1 | 1.03 | 0.00 |
| C16 | 22.64 | 30.76 |
| C16:1 | 5.42 | 6.77 |
| C18 | 6.02 | 7.43 |
| C18:1n-9 | 24.40 | 32.40 |
| C18:2n-6 | 15.63 | 12.29 |
| C18:3n-3 | 4.18 | 1.68 |
Romano, jade perch, Table 4
| Fatty acid | Fish oil diet | Tilapia muscle | Soybean oil diet | Tilapia muscle |
|---|---|---|---|---|
| C18:3n-3 | 1.11 | 0.82 | 5.78 | 2.57 |
| C20:4n-6 | 0.67 | 1.03 | * | 1.98 |
| C20:5n-3 | 11.38 | 2.66 | 0.88 | 0.22 |
| C22:6n-3 | 10.72 | 14.08 | 1.84 | 4.84 |
* not detected
One possibility of this finding could include a preferential use of omega-3 fatty acids as an energy source over SFA and MUFA. Another contributor may include the synthesis of SFA from dietary starches, leading to the relatively high lipid content of jade perch. This process may have been accelerated due to the elevated water temperatures, and thus feeding rates, in this study. Therefore, research on the potential effects of temperature and dietary starch on the lipid content in jade perch should be explored.
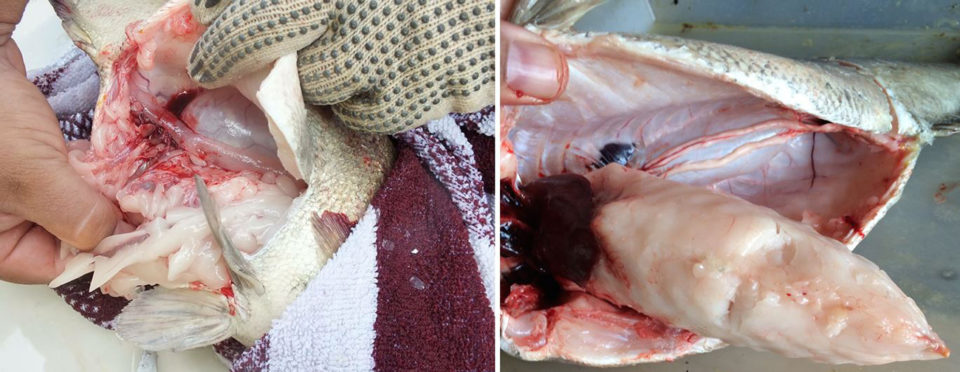
It is important to note that the sampled fish were still juveniles and not market size. Although the fatty acid composition is unlikely to be different compared to the older fish, it could be anticipated that the whole-body lipid content would be substantially higher. This is because it is commonly observed in our culture system that market size jade perch (>300 grams) store a great deal of internal fat (Fig. 5) that can be up to 30 percent of their actual weight. Again, the relatively high water temperatures could have influenced this.
One strategy to improve the fatty acid composition of fish can include the use of finishing diets that contain higher amounts of fish oil or microalgae oils that are rich in omega-3 fatty acids. This was shown to be relatively effective in jade perch (Van Hoestenberghe et al. 2013), however, these oils can be expensive and thus leave little incentive for farmers to adopt. Alternative strategies, such as biofloc technology or aquamimicry to encourage natural foods in the water, could be an area worth exploring to potentially enhance the nutritive value of jade perch.
Perspectives
Jade perch have many characteristics beneficial to aquaculture and this industry will likely continue expanding and spread to other countries. However, the suggestion that farmed jade perch is a fatty acid-rich product is not entirely accurate. Similar to other fish, jade perch would only be rich in omega-3 fatty acids if their diets also consisted of these nutrients in high amounts. Therefore, it would be misleading to suggest that farmed jade perch is a healthier option than other fish for consumption.
References available from first author.
Authors
-
Nicholas Romano, Ph.D.
Senior Lecturer
Aquaculture Department, Faculty of Agriculture
Universiti Putra Malaysia, Serdang, Malaysia -
Firas M. Al-Khafaji
Aquaculture Department, Faculty of Agriculture
Universiti Putra Malaysia, Serdang, Malaysia -
S.M. Nurul Amin, Ph.D.
Aquaculture Department, Faculty of Agriculture
Universiti Putra Malaysia, Serdang, Malaysia -
Mahdi Ebrahimi, Ph.D.
Department of Veterinary Preclinical Sciences, Faculty of Veterinary Medicine
Universiti Putra Malaysia, Serdang, Malaysia
Tagged With
Related Posts

Aquafeeds
Bridging the omega-3 gap with methane, microalgae
Innovation is leading to new ingredient options for renewable sources of omega-3 fatty acids. But Replicating long chain fatty acids is a tall order, Advocate contributor Lisa Duchene discovered.

Aquafeeds
Alternative feed ingredient universe to convene at F3 meeting
What started out as a simple yet ambitious contest to drive innovation in the aquafeed sector has evolved into a fully global competition – and collaboration – amongst ingredient suppliers and feed manufacturers.

Aquafeeds
Buggin’ out: Tapping the potential of insect meal in aquaculture
Black soldier flies are gaining interest as a leading alternative ingredient in aquafeeds. But will the “ick” factor be a turn-off? Advocate contributor Clare Leschin-Hoar investigates.

Health & Welfare
Aquamimicry: A revolutionary concept for shrimp farming
Aquamimicry simulates natural, estuarine production conditions by creating zooplankton blooms as supplemental nutrition to the cultured shrimp, and beneficial bacteria to maintain water quality. Better-quality shrimp can be produced at lower cost and in a more sustainable manner.


