Disinfection in semi-intensive shrimp ponds

In the Americas, many shrimp producers use large ponds and manage them for semi-intensive culture. The main pond bottom soil management practices used by these producers are pond dry-out and liming between crops.
There are three basic reasons for these practices: acceleration of organic matter decomposition, neutralization of soil acidity and destruction of unwanted organisms, including disease agents.
Pond soil moisture, respiration
Immediately after draining a pond, the pores in bottom soil are full of water that usually is depleted of dissolved oxygen, but in the dry pond bottom, pores and cracks in the soil fill with air that contains about 250 parts per million (ppm) oxygen. Aeration of soil as a result of drying increases the availability of oxygen for oxidation of organic matter by aerobic bacteria and chemical oxidation of reduced substances such as ferrous iron, manganous manganese, nitrite and sulfide present in soil at the end of the grow-out period.
The relationship between soil moisture concentration and respiration rate (organic matter decomposition) in three bottom soils is illustrated in Fig. 1. Respiration was low until soil moisture concentration fell below 30 percent, and highest rates of respiration were in the soil moisture concentration range of 10 to 20 percent. Respiration rate dropped quickly as soil moisture concentration fell below 10 percent, because there was insufficient moisture for optimal microbial activity.
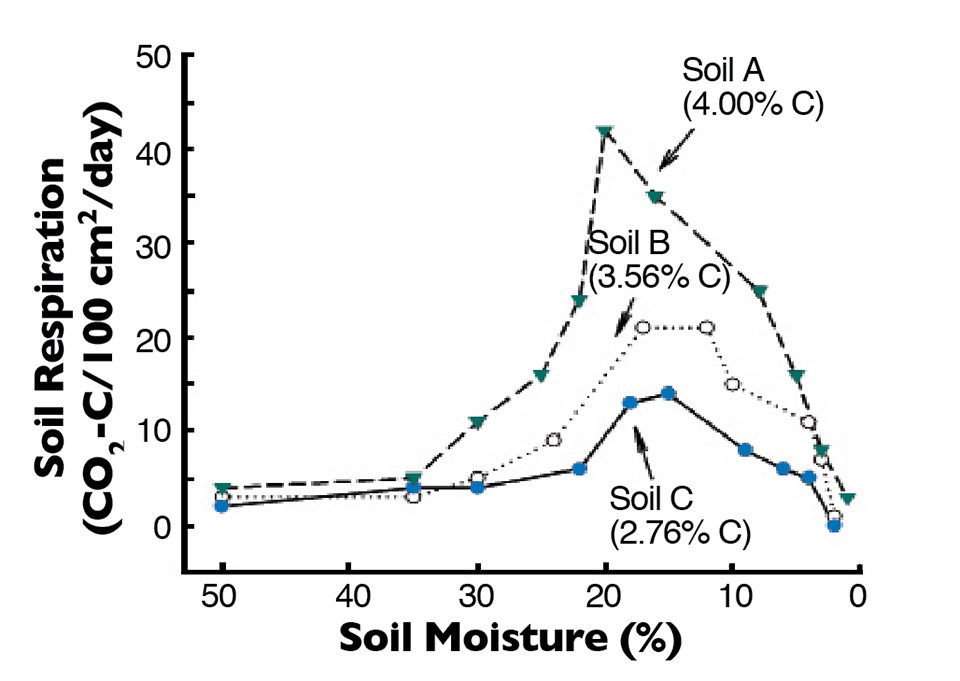
Larger aquatic animals that can survive in puddles of water die when puddles in pond bottoms dry up. Planktonic organisms and soil microorganisms also die from desiccation when soils become so dry that no biologically available water is present. Of course, some microorganisms form spores or cysts that survive desiccation for protracted periods, but pond dry-outs certainly lessen the abundance of soil microorganisms.
In a laboratory study, sediment from a recently drained pond (soil A of Fig. 1) was held at its optimum moisture concentration of 18 percent by periodic water additions. The respiration rate declined steadily as the more readily decomposable organic matter was used up (Fi. 2). The respiration rate after 30 days was only about 20 percent of the initial rate, and almost 80 percent of the total carbon dioxide released by respiration occurred within 20 days.
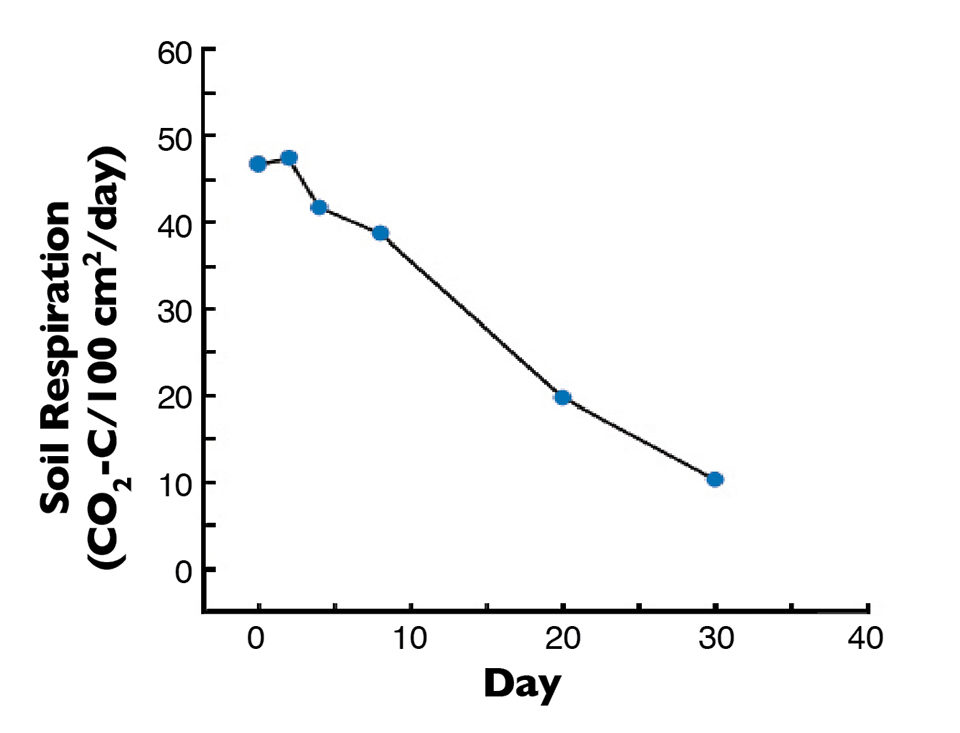
This suggested the readily oxidizable organic matter – the part of the organic matter that would be problematic during the next crop – was broken down very quickly. Moreover, most soils become too dry for appreciable microbial decomposition of organic matter within two to three weeks during dry weather. There therefore is not much need for drying pond bottoms more than three weeks.

Pond soil drying, tilling
Soils dry first on the surface, which creates an impediment to further evaporation and oxidation. Many soils break into columnar blocks, which facilitates drying and aeration, but the surfaces of the blocks dry and hinder evaporation and oxidation. Chemically reduced soil has a dark – often black – color because of the presence of ferrous iron. By digging into the soil surface or breaking columnar blocks of soil, one can observe soil color and ascertain if the soil has been oxidized.
Soil can be tilled to break its surface and pulverize clods to encourage drying and aeration. Probably the best implement for tilling pond bottoms is a disk harrow. It usually is sufficient to till to a depth of 10 to 15 cm, with the most effective practice to till the bottom twice, but in opposite directions. Of course, heavy clay soils benefit more from tilling than do lighter silt or sandy soils.
If excessive sediment accumulates in pond bottoms, soils will not dry completely, and tilling may not be possible because the wet sediment will not support the weight of the tillage equipment. Also, the soil may be so moist that it cannot be pulverized well. Where sediment is more than 10 to 15 cm deep, it often is advisable to remove it from pond bottoms to facilitate dry-out.
Soil pH, liming
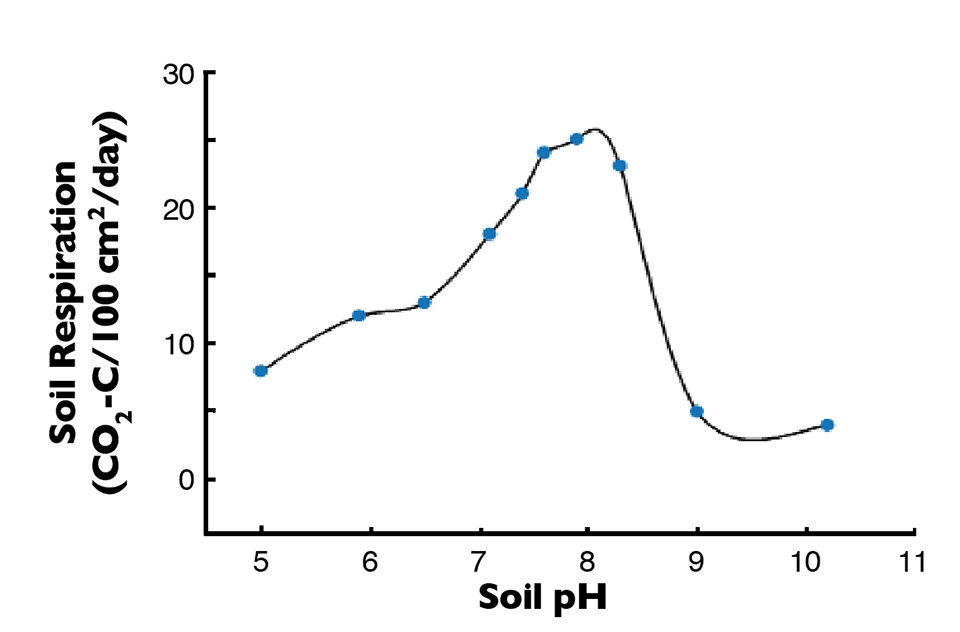
The pH level of pond soils has a pronounced effect on microbial degradation of organic matter, as illustrated in Fig. 3. Ponds with soil pHs below 7.5 should be limed to enhance the rate of decomposition. Liming can be accomplished by spreading either agricultural limestone (finely pulverized limestone) or lime (limestone that has been burned in a kiln to drive off carbon dioxide).
Lime is available as burnt lime that consists of oxides of calcium and magnesium or as hydrated lime produced by treating burnt lime with water to convert oxides to hydroxides. In terms of their abilities to neutralize acidity, burnt lime and hydrated lime are roughly 1.8 and 1.4 times stronger, respectively, than agricultural limestone.
Typical liming rates based on the use of high-quality liming materials in a soil containing about 30 percent clay are provided in Table 1. Liming rates should be decreased for coarser-textured soils or increased for finer soils at about 200 kg/ha/percentage unit of clay. However, pond managers seldom know the clay content of bottom soils, and the best approach probably is to use the amounts shown in Table 1 regardless of soil texture.
Li Li, Liming rates, Table 1
| Soil pH | Liming Rate (kg/ha) Agricultural Limestone | Liming Rate (kg/ha) Hydrated Lime | Liming Rate (kg/ha) Burnt Lime |
|---|
Soil pH | Liming Rate (kg/ha) Agricultural Limestone | Liming Rate (kg/ha) Hydrated Lime | Liming Rate (kg/ha) Burnt Lime |
|---|---|---|---|
| 4.5 or less | 6,000 | 4,500 | 3,400 |
| 4.6-5.0 | 5,000 | 3,700 | 2,800 |
| 5.1-5.5 | 4,000 | 3,000 | 2,200 |
| 5.6-6.0 | 3,000 | 2,200 | 1,700 |
| 6.1-6.5 | 2,000 | 1,500 | 1,100 |
| 6.6-7.5 | 1,000 | 750 | 600 |
| Above 7.5 | 0 | 0 | 0 |
(Editor’s Note: This article was originally published in the July/August 2014 print edition of the Global Aquaculture Advocate.)
Authors
-
Li Li, Ph.D.
College of Fisheries and Life Science
Shanghai Ocean University
Shanghai, China -
Julio F. Queiroz
Embrapa Meio Ambiente
São Paulo, Brazil -

Claude E. Boyd, Ph.D.
School of Fisheries
Aquaculture and Aquatic Sciences
Auburn University, Alabama 36849 USA
Related Posts

Responsibility
The importance of liming materials in aquaculture
Liming is an important management tool for preparing aquaculture ponds between and during production cycles. Several products are sometimes used in aquaculture for liming production ponds, including agricultural limestone, calcium silicate (CaSiO3) and sodium bicarbonate (NaHCO3).

Responsibility
Calcium and magnesium use in aquaculture
Aquatic plants and animals get the essential nutrients calcium and magnesium from water and food. Calcium concentrations impact the hydration and development of eggs in a hatchery, where calcium carbonate precipitation can be troublesome.

Health & Welfare
Decomposition and accumulation of organic matter in ponds
Aquaculture ponds accumulate organic matter from organic fertilizer, remains of microorganisms produced within the pond, feces of the culture animals and uneaten feed. Claude E. Boyd, Ph.D., details the leading organic matter management practices, and says that the accumulation of organic matter is often not as great as believed.

Responsibility
A look at various intensive shrimp farming systems in Asia
The impact of diseases led some Asian shrimp farming countries to develop biofloc and recirculation aquaculture system (RAS) production technologies. Treating incoming water for culture operations and wastewater treatment are biosecurity measures for disease prevention and control.


