Isolated phages evaluated effective in controlling major farmed shrimp disease, inhibiting bacterial growth

Acute hepatopancreatic necrosis disease (AHPND) is caused by a Vibrio bacterium (V. parahaemolyticus) that has caused substantial mortalities – up to 100 percent – in affected, farmed penaeid shrimp in several countries.
This disease was first reported in China in 2009 and subsequent outbreaks occurred in Malaysia, Thailand, the Philippines, Mexico and various other Latin America countries, and in 2017 also in Bangladesh and the United States. Losses due to AHPND have been estimated to be more than $1 billion per year. Therefore, it is important to develop and effectively implement control measures to prevent catastrophic losses to the shrimp farming industry.
Bacteriophages, commonly called phages, are ubiquitous viruses that infect bacteria and can be used to control infectious diseases in humans, animals and plants. The name is based on the word bacteria and the Greek phagein, meaning “to devour.”
Phages can replicate within bacteria after their genome is injected into the bacteria. Phages have been proposed as an alternative method since they show an effective bacteriolytic activity and possess advantages over conventional antibiotics: phages are natural and are most common and diverse, and widely distributed in the environment, including seawater – and are also relatively inexpensive. Phages have been used for many years as an alternative to antibiotics in several countries, and are a possible treatment against multi-drug-resistant strains of many bacteria resistant against several drugs.
Phage infectivity results
For bacteriophage pVp-1, its infectivity was tested in 22 strains AHPND-causing Vibrio parahaemolyticus (abbreviated as VpAHPND) strains. These bacterial isolates were obtained from pond water, sediment samples and the stomachs of shrimp affected by AHPND/EMS in Southeast Asia and Latin America countries. Pure cultures were obtained by streaking on 2 percent NaCl tryptic soy agar (TSA) plates. This phage was able to infect 91 percent (20 strains) of the VpAHPND tested and demonstrated strong bacteriolytic activity against 3 highly pathogenic strains (Fig. 1).
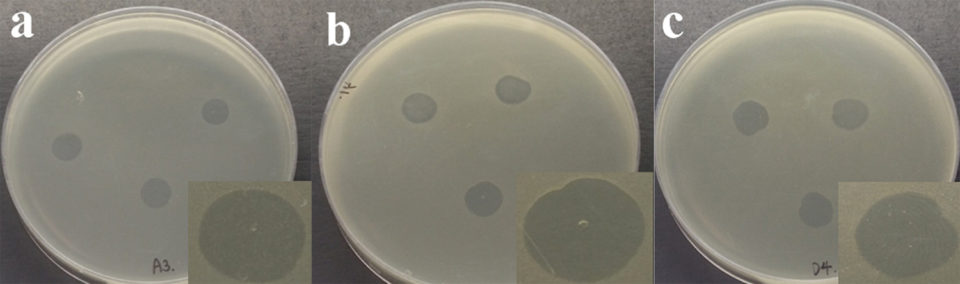
Evaluating effectiveness
Furthermore, its effectiveness was evaluated in the laboratory challenge studies with SPF (specific pathogen free) juveniles of Pacific white shrimp (Penaeus vannamei). Test animals (n = 96, average weight = 1.02 g) were kept under appropriate conditions (water temperature 25 degrees-C; salinity 25 percent) and three tanks were designed for controls.
Tank 1 was designated as a negative control without bacterial challenge or phage treatment; tank 2 was designated as a phage control with phage treatment by bath immersion (1.5 x 106 PFU/ml) and feeding (1.5 x 108 PFU/shrimp) using pellets (5 percent of body weight) that had been impregnated with the phage suspension, but not bacterially challenged. And tank 3 was designated as a positive control with a bacterial challenge but not phage treated.
For the challenge test, shrimp were treated with at various time points (24, 6, and 1 hour prior to the bacterial challenge, and 1 hour after the bacterial challenge), and exposed to V. parahaemolyticus 13-028/A3 (5.0 x 105 CFU/ml) for 24 hours by immersion method. Each group was monitored for symptoms of infection and cumulative mortality was recorded daily for five days after the bacterial challenge.
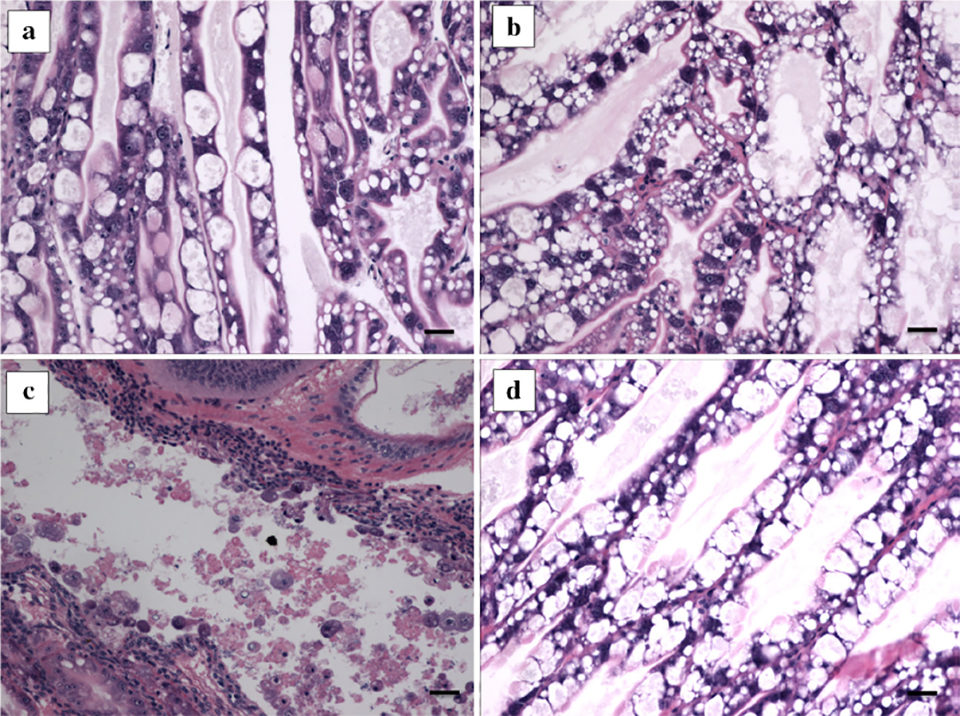
From the results, shrimp treated with pVp-1 displayed significant protection, over 25 percent (maximum 50 percent mortality), whereas the positive control groups (not treated with phage pVp-1, only exposed to VpAHPND) showed a 100 percent mortality. Histopathological features of the hepatopancreas of shrimp were shown in Fig. 2.
V. campbellii strains carrying pirABvp genes from diseased shrimp were recently identified as causative agents of AHPND, and we tested these strains for the second bacteriophage, pVp-2, isolated from Penaeus vannamei. The phage pVp-2 effectively lysed several Vibrio parahaemolyticus (VpAHPND)and also Vibrio campbellii (VcAHPND) and forming plaques in TSA+ plates (Fig. 3).
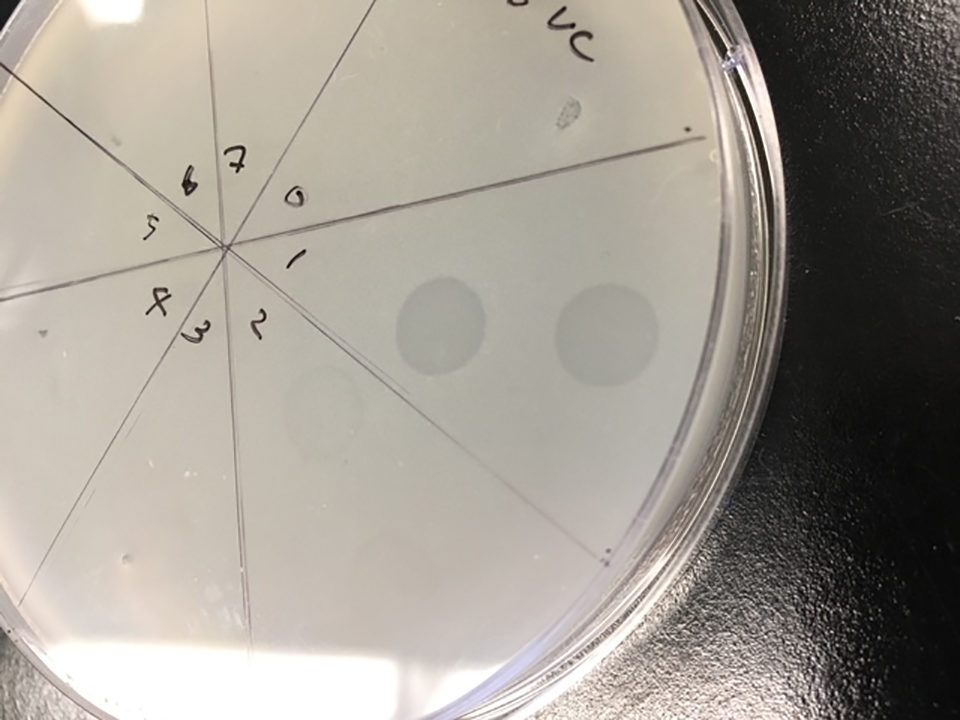
Perspectives
In our study, we demonstrated that the isolated phages evaluated are effective in controlling AHPND infection and inhibit bacterial growth when applied to the shrimp. Further studies are needed to evaluate the effectiveness of the bacteriophages against AHPND in laboratory and field trials.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-

-

Kathy F.J. Tang, Ph.D.
Yellow Sea Fisheries Research Institute
Chinese Academy of Fishery Sciences, China -

Angela Corbin, M.S.
Assistant Professor
Department of Biological Science
Nicholls State University, USA
Tagged With
Related Posts

Innovation & Investment
Scottish firm honing bacteriophages into aquaculture-disease assassins
Scottish biotech firm Fixed Phage aims to bottle the powers of bacteriophages to deploy these “bacteria killers” on some of the world’s most destructive aquaculture diseases.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Health & Welfare
Four AHPND strains identified on Latin American shrimp farms
Two virulence genes are known to encode a binary photorhabdus insect-related toxin that causes acute hepatopancreatic necrosis disease in shrimp. The pathogenicities of these V. campbellii strains were evaluated through laboratory infection and subsequent histological examination in P. vannamei shrimp.

Health & Welfare
Antibiotic-resistant bacteria, part 1
No antimicrobial agent has been developed specifically for aquaculture applications. However, some antibiotic products used to treat humans or land-based animals have been approved for use at aquaculture facilities.

