Study sees positive effects on immune response, disease resistance

Biofloc technology (BFT) has been amply studied and contributes to the maintenance of adequate water quality in aquaculture systems and to the nutrition of farmed aquatic animals. It has been shown that BFT systems not only keep ammonia below toxic levels and improve the efficiency of nutrient utilization of farmed animals, but also provide additional nutrients and exogenous digestive enzymes. BFT application can also support greater growth, survival and reproductive performance of the cultured animals.
Few studies have investigated the immunological potential of BFT systems, although it is widely known that microorganisms, their cellular components or their metabolites can act as immunostimulants that improve the innate immune system of shrimp and provide better protection against pathogens. Therefore, immunological stimulation can be a very important characteristic in shrimp cultivated with biofloc and contributing to the control of diseases. For example, it could partly explain the lower prevalence of Acute Hepatopancreatic Necrosis Disease (AHPND) observed in shrimp farms that apply BFT. This disease has resulted in major losses for the farmed shrimp industry in many countries.
This article – adapted and summarized from Aquacultura (Ecuador) Issue 128, April 2019 – reports on a study with shrimp cultivated in biofloc systems supplied with different sources of organic carbon (molasses, tapioca, tapioca byproducts and rice bran) to determine possible different immune system stimulation and disease resistance.
Study setup
The experimental culture setup included 20 glass tanks (90 x 40 x 35 cm) filled with 100 liters of seawater and used as experimental culture units. Water was continuously aerated, temperature was maintained at 27.3 to 28.3 degrees-C, and the light regime was 12 hours light-12 hours dark. Juvenile Pacific white shrimp (Litopenaeus vannamei) with an initial average body weight of 2.02 ± 0.05 grams were randomly stocked at 30 animals per tank (83 shrimp per square meter). Shrimp were fed four times per day a commercial, 30 percent crude protein pellet for 49 days, with feeding levels of 7 percent wet body weight per day and feed amounts adjusted according to tank biomass.
The experiment used five treatments (four replicate tanks per treatment), including a control treatment without addition of organic carbon and with a 50 percent weekly water exchange; and four treatments with different organic carbon sources added for biofloc development (molasses, tapioca, tapioca byproducts and rice bran, respectively). Fresh water was added regularly only to compensate for water loss due to evaporation. All sources of organic carbon were purchased locally. The organic carbon was added daily two hours after feeding at an estimated C:N ratio of 15. Water quality was monitored for the entire period of 49 days in biofloc systems supplied with different sources of organic carbon (molasses, tapioca, tapioca byproducts and rice bran). Shrimp growth performance, immune responses and resistance to infectious myonecrosis virus (IMNV) were also verified.
For additional information on the experimental setup; water quality; production and shrimp yield; protein and lipid assimilation; IMNV test; immune parameters; bacterial counts; and statistical analyses, please consult the original publication.
Results and discussion
In this research, we show that the application of biofloc technology for the cultivation of Pacific white shrimp significantly affects the immune response of shrimp, which can increase the robustness of shrimp to resist infection. These effects appear to be independent of the type of carbon source used to grow the biofloc.
Each type of biofloc grown with a different carbon source (molasses, tapioca, tapioca byproduct, rice bran) was adequate to maintain the general parameters of water quality in a normal range for shrimp growth. The lower level of dissolved inorganic nitrogen generally observed in the biofloc-treated tanks compared to the control with regular water replacement confirmed previous reports on the effect of BFT application on water quality in shrimp culture. Other research elsewhere has evaluated the different effects of simple carbohydrates vs. more complex carbohydrates that are applied as a carbon source in biofloc ponds. Simple sugars, such as sucrose, result in faster elimination of ammonia, while more complex carbohydrates require more time to break down into simple sugars, resulting in a slower removal of ammonia.
This may explain the higher levels of TAN at several times during our experimental period for the rice bran treatment, the carbon source that contained the highest level of fiber, compared to the other carbon source treatments. In this sense, the fermentation of complex carbon sources before their application in the biofloc system could be an alternative solution and an interesting topic for further research on the use of complex or fibrous carbon sources. In previous studies, it was shown that the application of BFT in general results in higher growth performance, FCR and survival of farmed shrimp. In our study, there also appears to be a trend towards a slightly higher growth and survival rate for shrimp in biofloc systems, although no significant differences were observed compared with shrimp from the control treatment. In addition, no differences were observed between the different treatments of carbon sources. The different sources of organic carbon, however, seemed to have an effect on the assimilation of proteins and lipids by the shrimp.
The assimilation of proteins by shrimp in biofloc treatments was greater than for control shrimp, but only significantly in the tapioca and rice bran treatments. A similar observation was made for the assimilation of lipids, which was significantly higher for the treatment of molasses and tapioca. Since the shrimp in all treatments received the same dietary supplementation of proteins and lipids contained in the commercial diet, and because only small correlations were observed between shrimp protein and lipid assimilation and the content of proteins and lipids in the carbon sources, the various assimilation values were the result of the uptake of proteins and lipids as biofloc biomass.
One of our recent studies showed that, depending on the composition of essential amino acids, biofloc can be considered a source of good quality proteins. Other authors have suggested that the improvement in protein assimilation by animals raised in BFT systems is also related to the increase in the activity of digestive proteinase in the intestinal tract as a result of the contribution of both exogenous digestive enzymes by microbes in the biofloc and the production of endogenous digestive enzymes stimulated by the biofloc.
The improved assimilation of proteins and lipids in the biofloc treatments clearly showed a positive contribution of the biofloc biomass generated from nutrient residues as a food source for the farmed animal. This, in turn, may result in a lower feed conversion factor in BFT systems, which was also observed in our study. In addition, the input-output relationship that represents the gain in shrimp biomass relative to the combined input of feed and carbon sources shows the effectiveness of the organic carbon source in relation to the biomass gain. In this sense, it can be observed that the use of rice bran as a source of organic carbon was the least effective compared to the other carbon sources in our study.
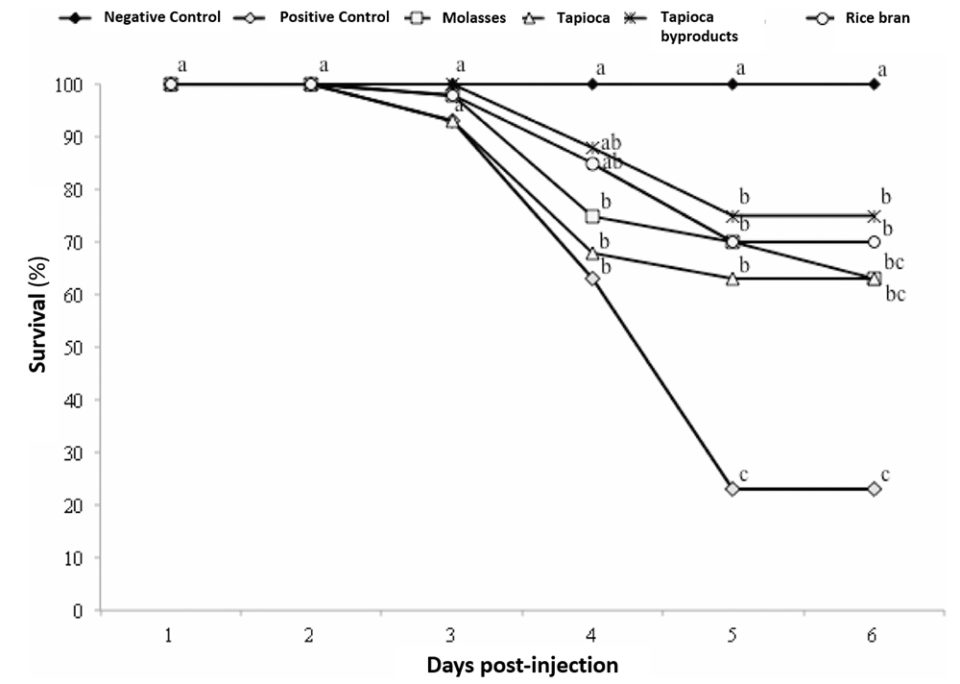
The biofloc clearly affected the innate immune response of the shrimp. For shrimp reared in a BFT system, the total hemocyte count and the phenoloxidase activity before the assay showed higher values compared to the control. This stimulation effect seems to be a general characteristic of biofloc, although the degree of stimulation seems to depend on the carbon source. The circulating hemocytes of crustaceans and other invertebrates are essential for immunity, since they perform functions such as phagocytosis, encapsulation and storage and release of the prophenoloxidase system.
Phenoloxidase (PO) is an enzyme of the defense mechanisms of crustaceans that leads to the melanization of foreign cells to inactivate them and prevent their spread throughout the body. This enzyme is highly stimulated by components of the microbial cell wall, such as lipopolysaccharides (LPS) and b-1,3-glucans. With shrimp grown in BFT-based systems, they consume microbial biofloc in situ, increasing the total number of hemocytes and PO activity and suggesting a stimulating effect of the consumed biofloc on shrimp immunity. When considering the stimulation of PO activity based on biofloc, authors have clearly demonstrated that the levels of expression of various genes that regulate the PO activation systems were significantly higher in shrimp cultured with biofloc compared to shrimp in a control treatment.
The absence of significant effects in terms of respiratory burst (RB) activity – a measure to determine the generation of reactive oxygen species associated with phagocytosis by shrimp hemocytes – indicates that phagocytosis might not occur more times when compared to the control. Therefore, it may be that the immune system is stimulated by an as yet uncharacterized variety of immunostimulatory microbial cell wall material, which results in a greater capacity for immune response. Other authors have reported similar observations, describing the effects of biofloc stimulating the release of hemocytes in the circulatory system, but also that antibacterial and bacteriolytic responses were not significantly affected.
After the IMNV test, decreased in the activity levels of total hemocyte count, PO and RB were observed for the positive control, which is a normal physiological response in case of infection. Recovery of the shrimp’s immune system from viral infections, if not fatal, can take a long time. Although the total hemocyte count level in all treatments tested was similar six days after infection, the higher levels of PO and RB activity for biofloc treatments as compared to the positive control pointed to a faster recovery, or a more constant activity of the immune system in these treatments. The increased activity or efficiency of the immune system of the shrimp from biofloc treatments was also illustrated with the survival of the shrimp after infection, which was significantly greater than in the positive challenge control.
It is also interesting to observe that the use of BFT in shrimp culture produces similar effects in terms of growth, food efficiency, inhibition of pathogenic bacteria, and immune responses like probiotic application. Various authors have reported that the addition of Bacillus subtilisto growing water or feed of white shrimp resulted in better shrimp growth and survival, inhibition of Vibrio growth in the shrimp gut, increased protease and amylase activities, as well as positive regulation of genes related to the immune system. And the mechanisms through which probiotic bacteria affect the performance of shrimp have been reviewed by several authors.

These include immunomodulation, competitive exclusion, bioremediation, provision of nutrient sources and enzymatic contribution to digestion, and blockage of quorum detection. The effects that biofloc can have for shrimp culture as shown in this study, as well as in other recently reported studies, strongly suggest that the beneficial effects associated with biofloc are at least in part parallel to those observed with the addition of probiotics.
Perspectives
This study provided information on the immunostimulatory nature of the biofloc for shrimp, and how this varies depending on the source of carbon supplied. It showed that biofloc has positive effects on the immune response of Pacific white shrimp and can result in increased resistance to the IMNV virus. Only slight differences were observed between the different organic carbon treatments. Overall, this study shows the potential for the application of BFT for the control and management of diseases in the shrimp farming industry.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Julie Ekasari, Ph.D.
Department of Aquaculture
School of Fisheries and Marine Sciences
Bogor University of Agriculture, Indonesia -
Muhammad Hanif Azhar, Ph.D.
Department of Aquaculture
School of Fisheries and Marine Sciences
Bogor University of Agriculture, Indonesia -
Enang H. Surawidjaja, Ph.D.
Department of Aquaculture
School of Fisheries and Marine Sciences
Bogor University of Agriculture, Indonesia -
Sri Nuryati, Ph.D.
Department of Aquaculture
School of Fisheries and Marine Sciences
Bogor University of Agriculture, Indonesia -
Peter De Schryver, Ph.D.
Aquaculture Laboratory and Artemia Reference Center
University of Ghent, Belgium -
Peter Bossier, Ph.D.
Aquaculture Laboratory and Artemia Reference Center
University of Ghent, Belgium
Tagged With
Related Posts

Aquafeeds
Biofloc and clear-water RAS systems: a comparison
This study compared two types of indoor, shrimp culture systems: clear-water RAS and biofloc systems. Clearwater RAS had the edge in water quality, but shrimp in the biofloc treatment had a higher feed conversion ratio.

Health & Welfare
Biofloc technology holds potential for carnivorous fish species
Juvenile carnivorous African catfish performed well in biofloc-based systems, which could help produce better quality and more disease-resistant seed of this important aquaculture species and support the expansion of African catfish farming industry.

Responsibility
Advances in super-intensive, zero-exchange shrimp raceways
Research at the Texas AgriLife Research Mariculture Laboratory is investigating ways to improve the economic viability of super-intensive raceways for shrimp production.

Responsibility
Bacterial amendments in shrimp grow-out ponds
Pond microbial communities are a critical and often overlooked component of aquaculture ecosystems. Bacterial amendments like probiotics provide significant support to shrimp farmers around the world.


