Weekly Vibrio monitoring key to predicting bacterial disease outbreaks

Vibrio infections are an increasingly common problem in intensive shrimp culture. Recent outbreaks around the world in both traditional and biofloc systems are significantly reducing production and proving to be a limiting factor in the further development of recirculating aquaculture and super-intensive, biofloc-dominated shrimp culture systems.
Many Vibrio species are ubiquitous in shrimp culture water, but do not negatively impact shrimp unless a stressor is present. To better understand Vibrio dynamics during a shrimp production cycle, the authors monitored Vibrio populations during nursery and grow-out phases in super-intensive, zero-exchange, biofloc-dominated raceways.
Trial setup
Nursery and grow-out trials were conducted in two, 100-m3 greenhouse-enclosed raceways with 1.1-meter mean water depth. Each raceway was equipped with two optical dissolved-oxygen probes and an in-line D.O.-monitoring system. Aeration, mixing and circulation were generated by 14 high-pressure injectors driven by up to two, 2-hp pumps per raceway. Solids concentrations were maintained within the desired range using a settling tank and foam fractionator for each raceway.
For the nursery phase, raceways were stocked with 5- to 10-day-old Litopenaeus vannamei postlarvae weighing 0.93 ± 0.56 mg from specific pathogen-free, Taura-resistant and fast-growth hybrid broodstock. Two days prior to stocking, the raceways were filled with 90 percent 10-ppm chlorinated natural water and 10 aged seawater at a salinity of 30 ppt. In addition to manually mixing every second day for the first three weeks, injectors were operated in the raceways at full flow for five minutes twice daily. Water flow to these injectors was gradually increased over time.
The postlarvae were fed a combination of a commercial microencapsulated artemia replacement and dry postlarvae feed for the first eight days post-stocking, and then appropriately sized particles with an immune booster and nutritional supplement for the remainder of the nursery phase. A commercial nitrifying bacteria product and white sugar were used to enhance nitrifying and heterotrophic bacterial activities to control nitrogen species. At the conclusion of the 62-day nursery phase, shrimp were harvested, weighed and held for restocking.
For the grow-out phase, biofloc-rich water from the nursery phase was reused to make up 87.5 percent of the raceway volume and supplemented with 12.5 percent disinfected seawater. Shrimp harvested from the nursery phase at about 6.45-gram weight were restocked into the two raceways at a density of 458/m3. No water exchange was conducted during either phase. Municipal freshwater was used to compensate for water losses due to evaporation and biofloc control.
The shrimp were fed a 40 percent-protein commercial feed and harvested after 38 days. Feed was supplied continuously by belt feeders. Feed particle sizes and rates were adjusted on an ongoing basis according to twice-weekly growth sampling and weekly checks of shrimp size variation, assumed growth, feed conversion and survival. A commercial probiotic was added every third day during the nursery phase and daily during the grow-out phase. Vibrio concentrations in the culture water were monitored twice weekly using thiosulfate citrate bile salts sucrose (TCBS) agar plates throughout both phases and Vibrio chromogenic agar plates late in the grow-out. Vibrio from the hemolymph and hepatopancreas of moribund shrimp was also cultured using these methods.
Samocha, L. vannamei performance, Table 1
| Raceway 1 | Raceway 2 |
|---|
Raceway 1 | Raceway 2 | |
|---|---|---|
| Average weight (g) | 6.49 | 6.43 |
| Maximum weight (g) | 11.90 | 10.50 |
| Minimum weight (g) | 0.60 | 0.50 |
| Coefficient of variation | 35.6 | 31.0 |
| Yield (kg/m3) | 3.43 | 3.28 |
| Feed-conversion ratio | 0.81 | 0.81 |
| Survival (%) | 97.8 | 94.6 |
Results
At the conclusion of the nursery trial, survival was high at 95 to 98 percent, and the 0.81 average feed-conversion ratio was low (Table 1). Water quality variables were all within the range suitable for L. vannamei culture. Mean temperature, salinity, D.O. concentration and pH were 26.6 degrees C, 30.4 ppt, 6.7 mg/L and 8.1, respectively. Mean total ammonia nitrogen (TAN) and nitrite nitrogen (NO2.-N) values were 0.76-0.80 and 1.60-2.27 mg/L, respectively. Total suspended solids (TSS) remained below 511 mg/L for the duration of the trial.
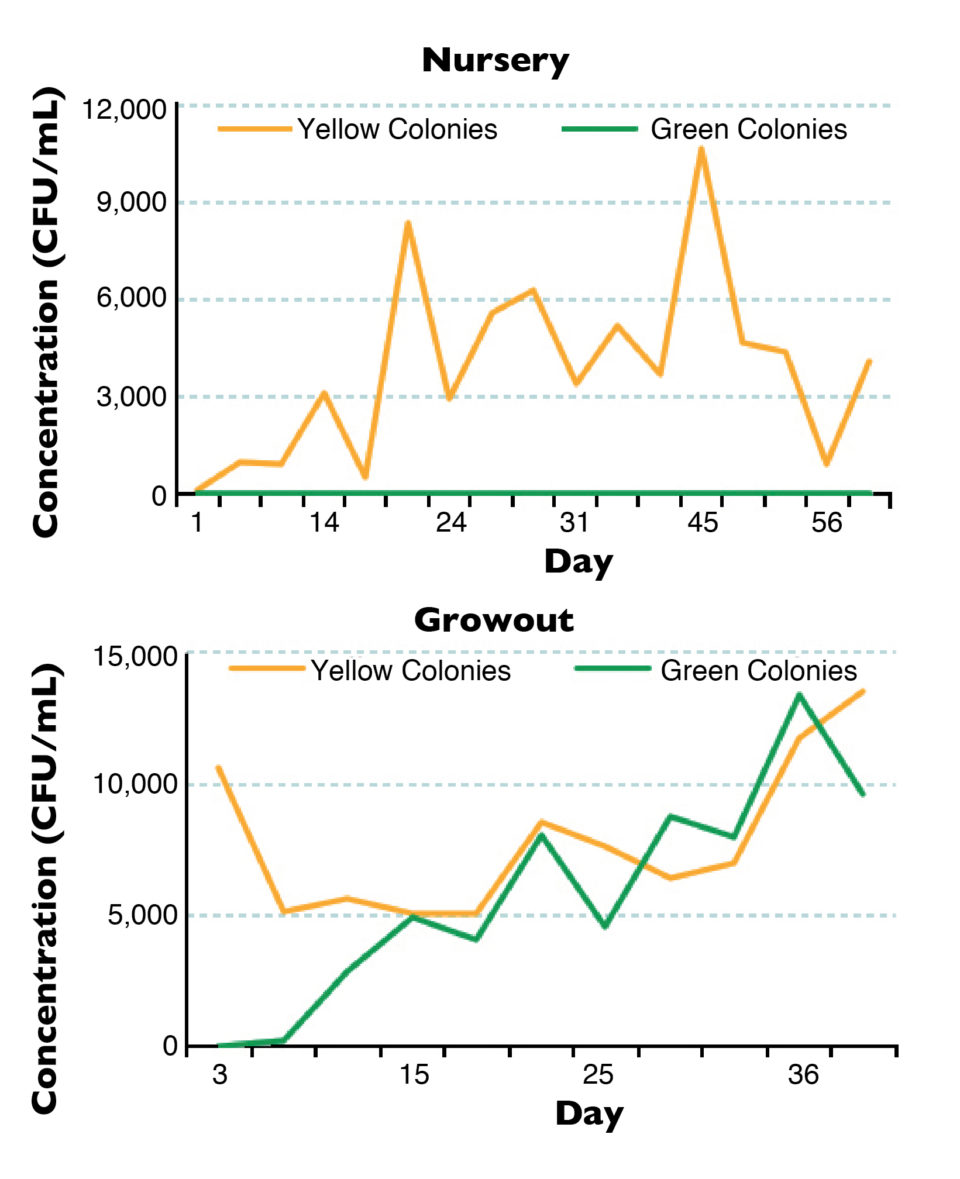
Non-sucrose-fermenting (green colony-forming units, GCFU) Vibrio concentrations remained below 50 CFU/mL – less than 9.1 percent of the total colony concentration throughout the trial – and were only observed on 12.1 percent of plates (Fig. 1). Sucrose-fermenting (yellow colony-forming units, YCFU) Vibrio concentrations were variable throughout the nursery trial, reaching concentrations as high as 12,200 CFU/mL (Table 2). There was no evidence of Vibriosis or chitinoclastic bacterial infections in either raceway.
During the nursery harvest and subsequent 24-hour holding period, the shrimp (particularly those from raceway 1) were subject to multiple stressors, including overcrowding, physical handling, low dissolved-oxygen levels, and high water temperature and suspended solids levels. The shrimp experienced approximately 20 percent mortality prior to restocking. Seven days into the grow-out phase, a new wave of shrimp mortalities commenced in raceway 1 and quickly spread to raceway 2.
Subsequent mortalities in both raceways resulted in early termination of the grow-out trial. At its conclusion, survival was 71.6 and 79.5 percent in the two raceways, with final shrimp weights of 18.37 and 19.01 g. Shrimp grew an average 2.2 and 2.3 g weekly, although feed-conversion ratios were relatively poor: 1.61 to 2.07 (Table 3).
Water quality parameters were within the ranges suitable for L. vannamei culture. Mean temperature, salinity, D.O. and pH were 30.3 degrees C, 30.4 ppt, 6.1 mg/L and 7.6, respectively. Mean total ammonia nitrogen and nitrite nitrogen values were 1.09-1.32 mg/L and 0.15-0.21 mg/L, respectively. Nitrate levels increased over time, reaching as high as 187 mg/L. TSS remained below 600 mg/L during the trial. GCFU Vibrio concentrations started increasing in both raceways after day 4 and were generally similar in concentration to YCFU, reaching 14,300 CFU/mL.
The total Vibrio concentration increased as the trial progressed, reaching 27,750 CFU/mL. Results suggested the presence of Vibrio parahaemolyticus, V. vulnificus and V. alginolyticus in the culture water and the hemolymph and hepatopancreas tissues of moribund shrimp. 16S rRNA sequencing confirmed the presence of these three species, along with V. harveyi and V. mytili in moribund shrimp hemolymph.
Samocha, Vibrio colonies, Table 2
| Nursery | Growout |
|---|
Nursery | Growout | |
|---|---|---|
| Yellow (x 1,000) | Mean 3.89 Range 0.09-12.20 | Mean 7.85 Range 3.45-17.70 |
| Green (x 1,000) | Mean 0.005 Range 0-0.10 | Mean 5.860 Range 0-14.30 |
| Total (x 1,000) | Mean 3.89 Range 0.09-12.20 | Mean 13.70 Range 5.30-27.75 |
| Green (%) | Mean 0.21 Range 0-1.77 | Mean 38.74 Range 0-72.00 |
Samocha, L. vannamei performance, Table 3
| Raceway 1 | Raceway 2 |
|---|
Raceway 1 | Raceway 2 | |
|---|---|---|
| Survival (%) | 79.5 | 71.6 |
| Final weight (g) | 18.37 | 19.01 |
| Growth (g/week) | 2.20 | 2.31 |
| Yield (kg/m3) | 6.02 | 6.92 |
| Feed-conversion ratio | 2.07 | 1.61 |
Perspectives
This study demonstrated the roles that multiple environmental and handling stressors play in triggering Vibrio outbreaks during transfers of shrimp from one production phase to the next, underscoring the importance of weekly Vibrio monitoring in predicting bacterial disease outbreaks. It further demonstrated how quickly bacterial diversity can change in a biofloc system. Even with additions of probiotics, environmental stresses can cause a shift to more pathogenic species.
These observations underscore the complexity of bacterial interactions within a biofloc system and warrant further investigation into avoiding and controlling detrimental Vibrio outbreaks in super-intensive, zero-exchange biofloc systems.
(Editor’s Note: This article was originally published in the May/June 2015 print edition of the Global Aquaculture Advocate.)
Authors
-
Tzachi M. Samocha, Ph.D.
Texas A & M AgriLife Research Mariculture Lab at Flour Bluff
4301 Waldron Road
Corpus Christi, Texas 78418 USA -
David I. Prangnell, Ph.D.
Texas A & M AgriLife Research Mariculture Lab at Flour Bluff
Leandro F. Castro, M.S.
Florida Organic Aquaculture
Fellsmere, Florida, USA -
Susan Laramore, Ph.D.
Harbor Branch Oceanographic Institute
Florida Atlantic University
Fort Pierce, Florida, USA
Tagged With
Related Posts

Intelligence
As ocean temperatures rise, so too will vibrio outbreaks
A study using a half-century of data has linked climate change and warming sea temperatures with an increase in illnesses from the common vibrio bacteria. Shellfish growers, fighting a particularly virulent strain of Vibrio parahaemolyticus, are changing their harvest protocols.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 1
Indian shrimp hatcheries have experienced larval mortality in the zoea-2 stage, with molt deterioration and resulting in heavy mortality. Authors investigated the problem holistically.

Health & Welfare
Cost-effective biosecurity crucial for shrimp farming
Many shrimp producers give only perfunctory attention to routine biosecurity at hatcheries and farms. A cost-effective biosecurity program for farmed shrimp requires reliable diagnostic tools to make timely decisions to control or exclude pathogens.


