Introduction could lead to healthy algal and beneficial bacterial blooms
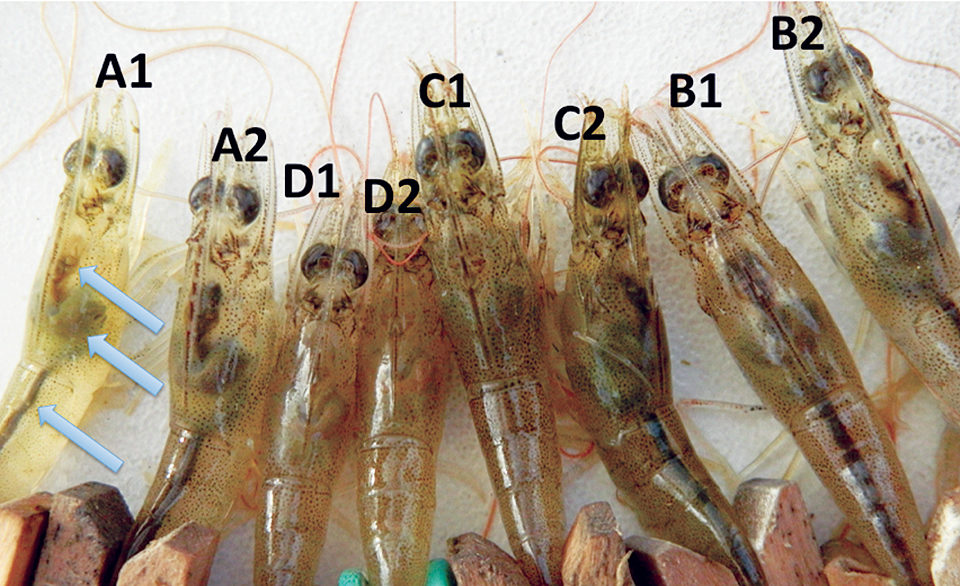
Ten days after exposure to pathogenic Vibrio parahaemolyticus, shrimp A1, A2, C2, B1 and B2 show normal stomachs,hepatopancreases and midguts (arrows from top to bottom). The remaining shrimp show signs of AHPND infection: empty stomachs, pale hepatopancreases and empty midguts.Several anecdotal reports have indicated that some antibiotic-free approaches such as polyculture and biofloc systems can reduce the risks of disease outbreaks at shrimp farms. Polyculture with tilapia has been known to confer some beneficial effects in controlling luminescent bacteria, Vibrio harveyi, affecting shrimp. Since V. parahaemolyticus, a virulent strain of which causes early mortality syndrome (EMS), is closely related to V. harveyi, polyculture of tilapia with shrimp may confer similar effects against this bacterial species.
Farm trials conducted in areas of Vietnam where EMS or acute hepatopancreatic necrosis disease (AHPND) is endemic have shown better survivability of shrimp grown in polyculture systems. However, lab experiments with controlled environments and standardized challenge models were needed to determine the effectiveness of this approach.
AHPND challenge study
A laboratory study was conducted at the University of Arizona to determine the effects of tilapia in controlling infection and mortality in Pacific white shrimp (Litopenaeus vannamei) due to a pathogenic Vibrio parahaemolyticus strain. Five treatments with three replicates each were used.
Treatment A was a negative control with culture tanks prepared without tilapia for 14 days prior to stocking with shrimp. For treatment B, tanks held Oreochromis niloticus tilapia for 14 days, after which the tilapia were removed prior to stocking of the shrimp. A 10-day AHPND challenge test followed after the addition of a bacterial suspension containing virulent V. parahaemolyticus that achieved a bacterial density of 3.105 cells/mL tank water.
In treatment C, tanks were prepared for 14 days with tilapia, then the tilapia were put into a suspended cage inside each tank prior to stocking of shrimp and a following AHPND challenge test. Treatment D used tanks prepared for 14 days without tilapia prior to shrimp stocking and following AHPND challenge. Treatment E was a positive control with tanks containing clear water at 20-ppt salinity prepared one day prior to stocking of shrimp and then followed by an AHPND challenge test.
Results
After 14 days of tank preparations to induce algal blooms and establish balanced biotic communities mimicking field practices in shrimp farming, bacterial counts revealed the bacterial density was not significantly different among treatments A, B, C and D. However, their counts were 3 logs higher than for the positive control.
Ten days after the addition of the bacterial suspensions in the challenge tests, survival rates were significantly different among treatments (Fig. 1). The survival rates for treatments A, B, C, D and E were 97.78 percent, 91.11 percent, 6.67 percent, 20.00 percent and 0 percent, respectively. The high survival rate in treatment A indicated the experimental conditions were suitable for the survivability of shrimp. Meanwhile, the zero survival in the positive control showed the high pathogenicity of the bacterial strain used in the study.
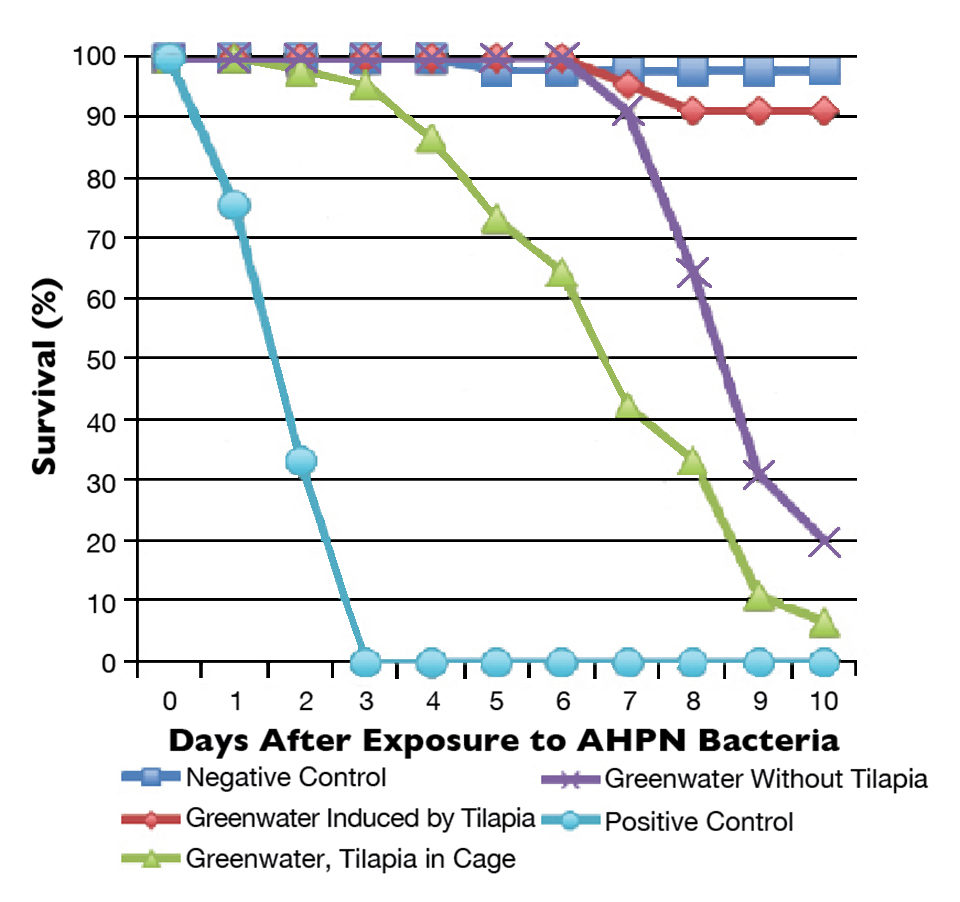
Although the AHPND bacteria were later isolated from the water and shrimp in all the challenged treatments, histological analyses showed that the infection rates and severities of the pathologies in different treatments corresponded to the survival rates. The bacteria counts in water samples showed a significant drop in treatments B, C and D compared to the bacterial density added by the challenged test. In contrast, bacterial density in the positive control using clear saline water showed a marked bloom of AHPND bacteria. This indicated that the native biota communities in water can interact with the AHPND bacteria and the infection caused by this strain.
The study suggested that the application of practices such as using tilapia in the reservoirs of shrimp farms to induce healthy algal and beneficial bacterial blooms in water prior to filling ponds could promote healthy, balanced biota communities in the pond water that could confer beneficial effects in controlling AHPND.
Discussion
Vibriosis diseases induced by luminescent bacteria have caused serious problems in shrimp farming. Some farming practices that took advantage of “greenwater” technology in which the green water was induced by tilapia could mitigate the luminescent vibriosis caused by Vibrio harveyi in Penaeus monodon.
Further works elucidating the mode of action of the greenwater technology have discovered that certain indigenous bacterial strains and algae in the greenwater had the ability to inhibit the growth of V. harveyi, explaining the mode of action of the greenwater technology or polyculture technology.
Perspectives
This study was the first step in demonstrating that the indigenous biota induced by tilapia or by tank preparation steps could lower the number of AHPND bacteria in water, thus delaying mortalities in challenged shrimp. However, an overbloom of algae could produce unexpected effects due to eutrophication and nutrient availability from dead algae that could benefit the AHPND bacteria. In addition, without competition from the indigenous biota, the AHPND V. parahaemolyticus could replicate in water to a level that causes infection.
These findings helped explain an observation that AHPND usually affected ponds that did not have algae or in which an excessive bloom of algae occurred or recently crashed.
(Editor’s Note: This article was originally published in the January/February 2014 print edition of the Global Aquaculture Advocate.)
Authors
-
Loc H. Tran, Ph.D.
School of Animal and Comparative Biomedical Sciences
Department of Soil, Water and Environmental Sciences
University of Arizona
1401 East University Boulevard
Tucson, Arizona 85721 USA -
Kevin M. Fitzsimmons, Ph.D.
Department of Soil, Water and Environmental Sciences
University of Arizona
1401 East University Boulevard
Tucson, Arizona 85721 USA -

Donald V. Lightner, Ph.D.
School of Animal and Comparative Biomedical Sciences
University of Arizona
1401 East University Boulevard
Tucson, Arizona 85721 USA
Tagged With
Related Posts

Health & Welfare
A look at tilapia aquaculture in Ghana
Aquaculture in Ghana has overcome its historic fits and starts and is helping to narrow the gap between domestic seafood production and consumption. Production is based on Nile tilapia.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Health & Welfare
Development of 1-monoglycerides against AHPND
1-monoglycerides are known for their antibacterial and antiviral effects in the human pharmaceutical and animal husbandry industries. As a substitution for the preventive use of antibiotics, these molecules are being evaluated in the fight against AHPND.

Intelligence
Aquaculture key to increasing seafood supplies to Arab states
Arab States have substantial natural resources to increase aquaculture production. Several types of systems are readily adaptable and can be implemented relatively quickly.


