An important variable around which operations are often timed

The atoms and molecules of a mass of matter to which energy is added vibrate faster and move slightly farther apart. The heat content of matter is a result of energy of the movements of atoms and molecules. Temperature is simply an indicator of how warm an object is as a result of its internal energy (or heat) content. It is a measurement that man developed and uses to assess how hot things are. A thermometer responds to the average kinetic energy of the molecules within a substance.
Sunlight is composed of photons (small particles) of energy. When sunlight passes through water, the energy in the light is transferred to water molecules increasing their heat content and causing the water to warm and its temperature to increase. Of course, energy also can be transferred to water by contact with a hot object, because energy (heat) in the hot object will be transferred to the cooler water by conduction.
Relevance of water temperature
The temperature of water is an important variable in aquaculture, but in most types of aquaculture it cannot be controlled and depends upon the amount of solar radiation, air temperature, or the temperature of water passing through the culture unit. Aquatic animals are strongly affected by temperature; aquaculture operations must be timed to correspond to water temperature, and temperature measurements are critical for efficient operations.
Temperature is an important factor effecting the growth and survival of all organisms. However, water temperature is especially important to the growth and survival of shrimp, fish, and other aquaculture animals, because they are poikilothermic (coldblooded). Poikilothermic animals cannot control body temperature, and they equilibrate with the temperature of the surrounding water. Aquaculture animals usually are classified as coldwater, warmwater, and tropical species.
Coldwater species will not tolerate temperatures above 20 to 25 degrees-C. Warmwater species will usually not reproduce at temperatures below 20 degrees-C or grow at temperatures below 10 to 15 degrees-C, but they survive much lower winter temperatures. Tropical species will die at temperatures of 10 to 20 degrees-C, and most do not grow at temperatures below 25 degrees-C.
Temperature ranges given above are very general, and each species, whether coldwater, warmwater, or tropical has its characteristic temperature requirements. The temperature effects on a tropical species of fish is illustrated in Fig. 1. There is a low temperature below which fish die, at slightly higher temperature, fish live, but they do not grow or grow very slowly. At a certain temperature, growth will increase rapidly with increasing temperature until the optimum temperature is reached. As temperature rises beyond the optimum temperature, growth will slow, cease, and fish will die if the increase continues.
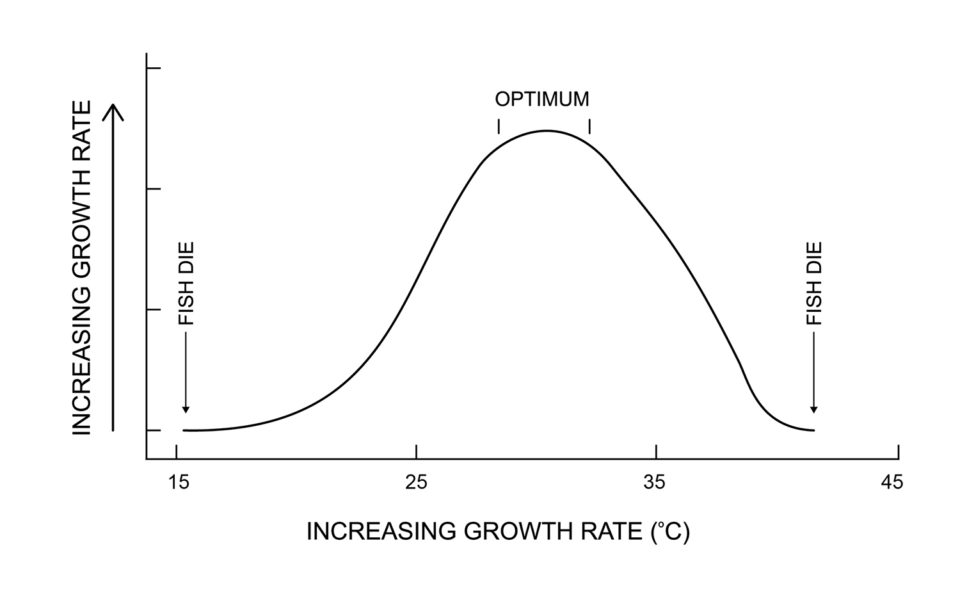
The relationship in Fig. 1 is slightly different for warmwater or coldwater species. These organisms are not likely to die as a result of low temperature in natural or aquaculture waters.
Effect on organism growth
Growth involves many metabolic and biochemical processes, and rates of these processes tend to increase in accordance with van’t Hoff’s law. This law states that chemical reactions will double or triple with each 10 degrees-C increase in temperature. As a result, in the temperature range within which growth increases rapidly with greater temperature, a 10 degrees-C increase in temperature will greatly increase growth rate. In biological applications, the van’t Hoff’s law effect usually is referred to as the temperature coefficient or Q10.
The growth pattern shown in Fig. 1 is for animals held in the laboratory under highly controlled conditions. In most aquaculture systems temperature cannot be controlled. There will be daily fluctuations in water temperature, seasonal trends in water temperature, and changes in water temperature related to weather patterns that may occur in any season. Moreover, culture animals may be stressed by factors other than temperature and grow slower than expected for the culture system water temperature.
A nice example of the above relationship is shown in Table 1 using data published years ago by Ji-Qiao Wang and colleagues. Carp were held under controlled temperature considered optimum for growth, but the salinity of the water was varied. Although temperature was near optimum, growth declined as salinity increased. Nevertheless, water temperature is a fundamental factor influencing growth, and aquaculture species should be selected to have temperature requirements allowing near optimum growth for the water temperature range at a particular production facility. Obviously, one cannot expect optimum temperature continuously, but periods with temperature deviating far from optimum will decrease production potential.
Boyd, Water Temperature, Table 1
| Salinity (ppt) | Food energy recovered as fish growth (%) |
|---|---|
| 0.5 | 33.4 |
| 2.5 | 31.8 |
| 4.5 | 22.2 |
| 6.5 | 20.1 |
| 8.5 | 10.4 |
| 10.5 | -1.0 |
Temperature has another important effect in ponds and in lakes for cage culture. The density of liquid water increases from 0 to 4 degrees-C and then decreases as temperature rises further. This results in ice floating, water bodies not freezing solid, and it causes surface water to heat faster and become less dense (lighter) than deeper water. This temperature-density dependence of water causes less dense surface waters, in which there is light for plants to produce oxygen through photosynthesis, to float on top of deeper, un-illuminated water.
This phenomenon, known as thermal stratification, separates the upper layer of water from deeper water, because wind action is not powerful enough to cause them to mix. Dead plankton settles to the bottom, but oxygen from the upper, illuminated layer cannot mix with the deeper layer because of thermal stratification. This results in dissolved oxygen depletion in the deeper water.
Destratification may occur as a result during cool periods or following intense rain and heavy wind that causes surface and bottom layers to mix. This phenomenon is commonly called on overturn, and the mixing of the deeper water with surface water can lead to dissolved oxygen depletion and fish mortality.
Aquaculture ponds usually are intentionally made shallow. They may stratify briefly during the day, but they mix at night when heat from the surface water is lost to the air by convection. Water currents caused by mechanical aerators in ponds also prevent thermal stratification.
Other effects of temperature
Temperature has other effects, because phytoplankton and zooplankton also respond to water temperature. Warmwater also favors greater rates of chemical reactions, and fertilizers and liming material applied to ponds will dissolve faster.
Dissolved oxygen concentration in water at equilibrium with air decreases as water temperature rises. This in itself is not troublesome to fish, because they respond to the pressure or percentage saturation of oxygen in water. Two freshwaters, one at 20 degrees-C containing 9.08 mg/L dissolved oxygen and the other at 32 degrees-C containing 7.29 mg/L dissolved oxygen are both saturated with dissolved oxygen. Although the cooler water contains more dissolved oxygen, both water are at 100 percent saturation.
Although the fact that warmer water holds less oxygen at saturation than does cooler water does not directly cause a problem, the respiration rate of all aerobic organisms increases with higher temperature. The culture species, phytoplankton, zooplankton and bacteria require more oxygen of respiration at higher temperature but from water that contains less dissolved oxygen. This is why dissolved oxygen depletion becomes a greater threat to the well-being of aquaculture animals as water temperature increases.
Temperature has some other impacts on ponds. Because warmer temperatures favor plankton growth, more carbon dioxide and bicarbonate will be removed from the water for use in photosynthesis than at lower temperature. This leads to greater fluctuation between nighttime and daytime pH, but usually this phenomenon does not affect the culture species appreciably.
There are several minor effects of temperature in aquaculture ponds that deserve mention. Warmer water has a lower viscosity than cooler water, and lower viscosity favors settling rates of suspended particles and seepage of water through pond soils. Suspended clay particles settle faster and more water seeps from ponds at higher water temperatures. Evaporation rate also increases with greater water temperature, and gas transfer by mechanical aerators also is favored by increasing water temperature.
Perspectives
As a general rule, physical, chemical, and biological processes in aquaculture systems are favored by an increase in temperature. However, biological processes are negatively impacted by temperatures above optimum, and stress and mortality of aquaculture animals and other organisms may result.
Author
-

Claude E. Boyd, Ph.D.
Professor Emeritus
School of Fisheries, Aquaculture and Aquatic Sciences
Auburn University, Auburn, Alabama 36849 USA
Tagged With
Related Posts

Responsibility
Ammonia nitrogen dynamics in aquaculture
The major sources of ammonia in aquaculture ponds are fertilizers and feeds, and problems with high ammonia are most common in feed-based aquaculture.

Responsibility
Assessing groundwater quality in aquaculture
Those interested in using groundwater for aquaculture should perform a thorough chemical analysis of the water. Several problems related to groundwater use in hatcheries and holding or transport vessels can be alleviated by degassing or aeration.

Responsibility
Calcium and magnesium use in aquaculture
Aquatic plants and animals get the essential nutrients calcium and magnesium from water and food. Calcium concentrations impact the hydration and development of eggs in a hatchery, where calcium carbonate precipitation can be troublesome.

Health & Welfare
Dissolved oxygen dynamics
Dissolved oxygen management is the most important requirement of aquaculture pond water quality. DO concentration below 3 mg/L is stressful to shrimp.

