Crucial to minimize risk spread of EHP/EHP-like pathogens

The White Feces Syndrome (WFS) refers to the presence of floating white fecal string in ponds rearing shrimp (Penaeus monodon & P. vannamei) in Southeast Asian countries. The syndrome is found to be associated with several problems at the farm level, including shrimp growth retardation, size disparities, reduced feeding and chronic mortalities.
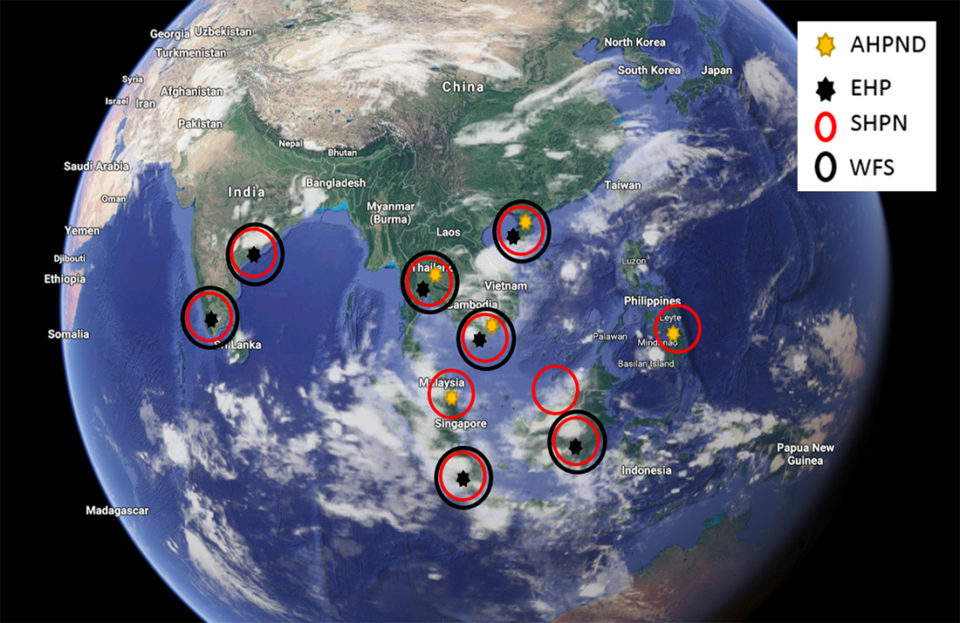
In countries where WFS has been reported, other enteric pathogens that also affect the hepatopancreas of shrimp have been reported as well. These include the microsporidian Enterocytozoon hepatopenaei (EHP), acute hepatopancreatic necrosis disease causing Vibrio parahaemolyticus (VpAHPND) and Septic Hepatopancreatic Necrosis (SHPN) caused by Vibrio sp. Fig. 1 shows Southeast Asian countries where WFS and other enteric diseases have been reported.
EHP, an intracellular microsporidian that replicates within the cytoplasm of the affected tubule epithelial cells in the hepatopancreas, is an emerging pathogen that affects mainly cultured shrimp P. vannamei in several Southeast Asian countries, including Indonesia, Vietnam, China, Thailand, India and Malaysia. The main clinical signs of EHP-infected shrimp are growth retardation, which leads to an increased variability in size. In a more advanced stage, EHP-infected shrimp typically display soft shells, lethargy, reduced feed intake and empty midgut. Histology of infected tissues reveals several developmental stages, including plasmodium and spore stages.
Several publications have attributed the cause of WFS to different etiologies, including Vibriocholera, gregarine-like organisms, Bacilloplasma sp. and Phascolarctobacterium sp. It has also been proposed that EHP is not the causative agent of WFS. Based on these discrepancies related to WFS, we conducted a systematic study to determine any potential relationship between WFS and enteric pathogens including EHP, in two different regions of the world where EHP has been reported.
The first part of the work was carried out in an Asian country with a history of EHP and WFS. Samples were collected from grow-out ponds, one displaying WFS and another where shrimp did not display WFS. Hepatopancreas and fecal string samples were collected and analyzed by histology and qPCR for EHP.

Hepatopancreas tissue from shrimp from each grow-out pond was individually analyzed by quantitative PCR (qPCR). Fig. 3 shows results of EHP loads in animals from ponds experiencing WFS vs. no WFS. The lower the Cycle threshold (Ct) value), the higher the EHP load in a sample.
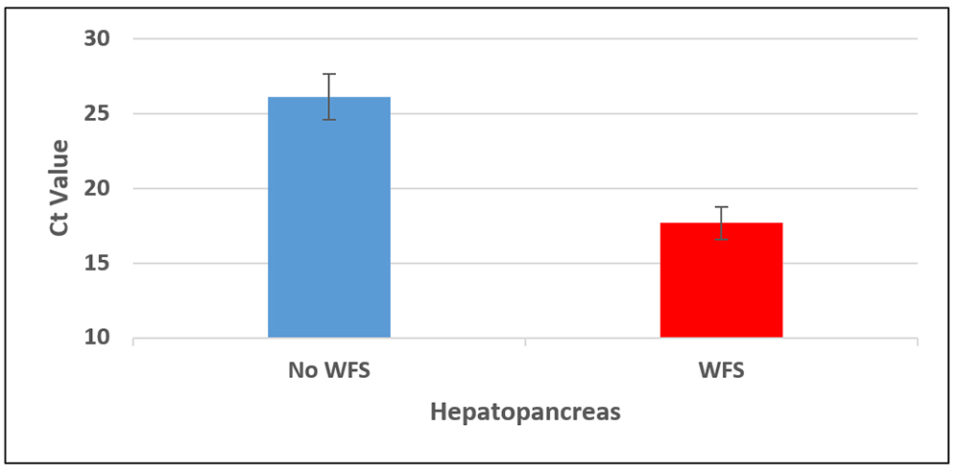
There is a clear difference in the EHP copy number between the two groups of animals. The average copy number in the WFS group was 4×107 copies per ul of DNA vs. 1×105 copies per ul DNA in ponds without WFS, which is more than 2 logs difference. This indicates that shrimp with WFS could be potentially more infectious of EHP than shrimp without WFS. The qPCR on the fecal strings of the same shrimp show similar results; i.e., shrimp displaying WFS presented a higher copy number of EHP in fecal strings than fecal strings of shrimp without WFS.
Later on, in the same EHP-endemic area, we determined the EHP copy number in samples from ponds with three different histories: ponds with no history of WFS; ponds displaying WFS; and ponds with a history of WFS. Fig. 4 shows the EHP copy number in animals from three different ponds.
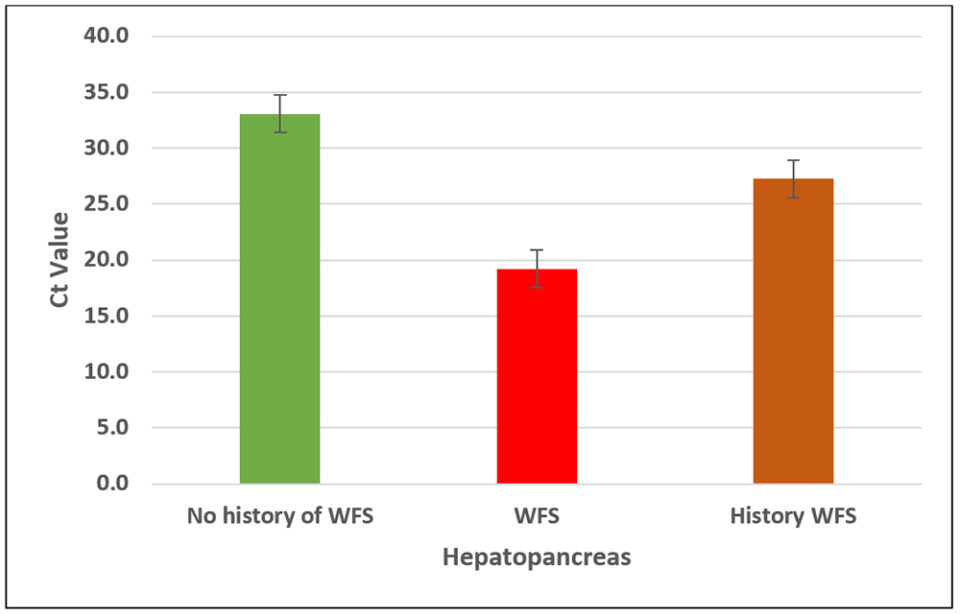
Once again, we found similar results to those obtained in the first experiment. The EHP copy number was higher in shrimp from ponds experiencing WFS (about 1×107 copies), followed by ponds with a history of EHP (about 4×104 copies) and ponds where WFS was not present nor any clinical sign of diseases were observed (about 4×102 copies).
WFS in the Americas
In the Western hemisphere, in 2016 we reported the presence of EHP and EHP-like pathogens in farmed shrimp. EHP-infected shrimp exhibited similar clinical signs to those displayed in Southeast Asian countries, including reduced feeding, severely retarded growth and size disparity. Two years later, we described the first case of WFS in areas where we previously reported the presence of EHP-infected Penaeus vannamei cultured in Latin America. The white fecal strings and the shrimp displaying white feces along the gastrointestinal tract are similar to those found in some Southeast Asian countries where WFS is present in EHP endemic regions.
Shrimp displaying WFS were analyzed by both H&E and PCR in order to determine the etiological agent associated to this pathology. The results of PCR and H&E are shown in Fig. 5.
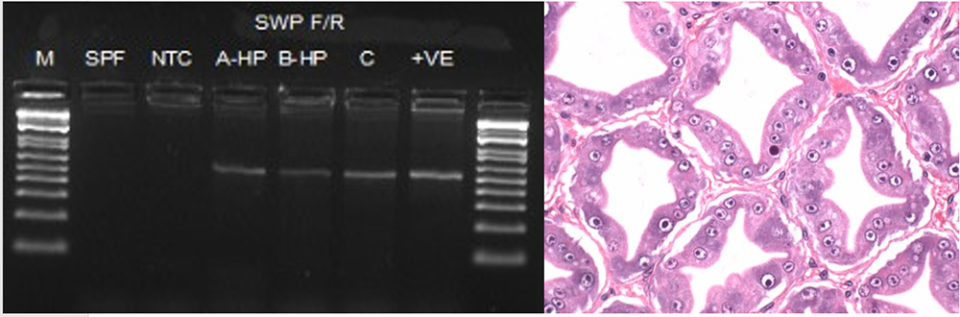
The histopathology of shrimp displaying WFS reveals the presence of plasmodium and spore stages typical of EHP (Fig. 5b). Samples examined by nested PCR to amplify the spore wall protein gene (SWP) confirmed the presence of EHP.
Role of SHPN in WFS
In both Southeast Asia and in Latin America where WFS has been reported, EHP is not the only pathogen associated with WFS. Shrimp analyzed by H&E show, in addition to EHP, lesions of Septic Hepatopancreatic Necrosis, or SHPN (Fig. 6).
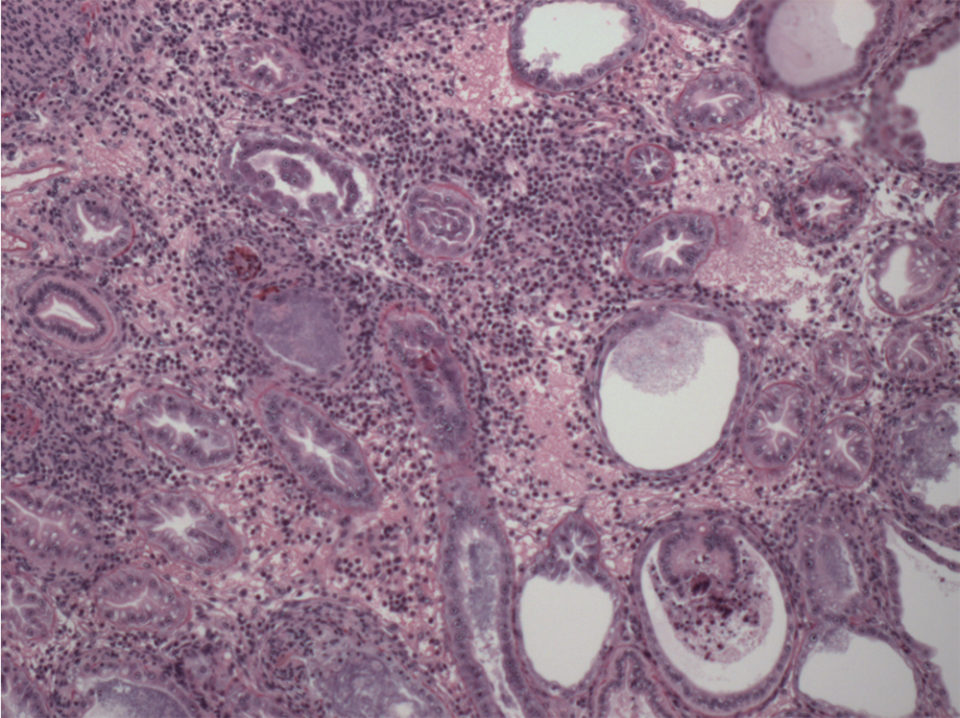
SHPN is a bacterial disease caused primarily by Vibrio spp., either pathogenic or opportunistic Vibrio spp. The opportunistic Vibrio are always in the hepatopancreas; however, when a primary enteric pathogen causes lesions in the hepatopancreas, these opportunistic Vibrio spp. get an entry to colonize the affected hepatopancreas and causing SHPN.
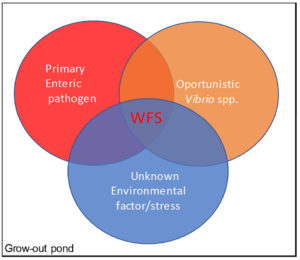
As Fig. 7 shows, and based on our analysis, we found an association among WFS, EHP and SHPN in grow-out ponds, which suggest that WFS is a possible physiological response in shrimp affected by a combination of enteric pathogens and possibly unknown environmental factor(s).
The finding of WFS in farmed shrimp in the Western Hemisphere is important, because it could be used as a predictor of the presence of EHP. In addition, it will help to elucidate the strong association between an EHP/EHP-like agent with the occurrence of white feces. As infection with EHP at early stages does not exhibit obvious clinical signs, shrimp cultured within the region should be monitored closely for the presence of EHP and white feces.
Perspectives
From our study, there is a strong association between White Feces Syndrome (WFS) and EHP in EHP-endemic regions. EHP in combination with other enteric pathogens (including SHPN) and a possible unknown factor(s) can cause White Feces Syndrome.
In EHP-endemic areas, shrimp displaying clinical signs of White Feces Syndrome indicate a very active EHP infection process in grow-out ponds. The White Feces Syndrome in the Americas shrimp industry could be a mirror of what happened in Southeast Asia 15 years ago, where EHP occurred sporadically but has now become one of the major sanitary risk factors for the shrimp industry in this region. Biosecurity strategies must be in place to minimize the risk of EHP/EHP-like pathogen spread in the Americas.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-

Luis Fernando Aranguren, Ph.D.
Corresponding author
Aquaculture Pathology Laboratory
School of Animal and Comparative Biomedical Sciences
University of Arizona
1117 E Lowell St.
Tucson, Arizona, 85721, USA -

Hung Mai, Ph.D.
Aquaculture Pathology Laboratory
School of Animal and Comparative Biomedical Sciences
University of Arizona
1117 E Lowell St.
Tucson, Arizona, 85721, USA -

Orlando Pichardo, B.Sc.
Escuela de Ciencias Aplicadas del Mar (ECAM)
Universidad del Oriente (UDP-NE) -

Bambong Hanggono
Fish Health and Environmental Laboratory
Brackishwater Aquaculture Development Center, Situbondo
PO. BOX 5 Panarukan Situbondo Jawa Timur – Indonesia 68351 -

Arun K. Dhar, Ph.D.
Associate Professor & Director
Aquaculture Pathology Laboratory
School of Animal and Comparative Biomedical Sciences
University of Arizona
1117 E Lowell St.
Tucson, Arizona, 85721 USA
Tagged With
Related Posts

Health & Welfare
Biosecurity principles for sustainable production using SPF shrimp
Basic components of biosecurity include knowledge of diseases, adequate detection methods and the use of “clean” shrimp stocks.

Health & Welfare
EHP a risk factor for other shrimp diseases
Laboratory challenges and a case-control study were used to determine the effects of EHP infection on two Vibrio diseases: acute hepatopancreatic necrosis disease (AHPND) and septic hepatopancreatic necrosis (SHPN).

Health & Welfare
Big shoes to fill: Dhar takes reins at shrimp pathology laboratory
Arun Dhar, Ph.D. will attempt to fill the “big shoes” of Dr. Donald Lightner at the University of Arizona’s Aquaculture Pathology Laboratory, where the shrimp disease EMS was diagnosed.

Health & Welfare
Development of 1-monoglycerides against AHPND
1-monoglycerides are known for their antibacterial and antiviral effects in the human pharmaceutical and animal husbandry industries. As a substitution for the preventive use of antibiotics, these molecules are being evaluated in the fight against AHPND.

