Significant effect at of 3.0 grams per kilogram
In shrimp aquaculture, the degradation of organic matter produces several metabolites, especially nitrogenous ones like ammonia and nitrite. Ammonia is also the main excretion product of crustaceans. At relatively low concentrations, ammonia – especially its nonionized form NH3 – is toxic for many aquaculture organisms including shrimp and can damage their organs. Low oxygen levels increase the negative effects of this metabolite.
Several strategies can be used to avoid or minimize high concentrations of ammonia in aquaculture ponds and/or effluents. These include the adequate management of water exchange and aeration, reduction of nitrogen inputs from feed, removal of pond sludge, biological treatments such as polyculture, and the use of mangrove forests as nitrogen sinks.
Most recently, products added to the water column or feed have been used to trap or transform ammonia into innocuous metabolites. In a study, the authors evaluated the effects of one of these products, BioAquaTM, an extract of Yucca schidigera, on water quality and the production parameters of Pacific white shrimp (Litopenaeus vannamei).
Experimental setup
Twelve 4.2-cubic-meter plastic pools were each stocked with 100 juvenile L. vannamei averaging 1 gram in weight. A reference diet was formulated and prepared at the food technology laboratory of the University of Sonora. Four treatments consisting of different levels of inclusion of the yucca extract in the reference diet were evaluated by triplicate in the pools. The levels of inclusion were 0 grams per kilogram (control), 1.0 grams per kilogram (treatment 1), 2.0 grams per kilogram (treatment 2), and 3.0 grams per kilogram (treatment 3).
Feed was supplied twice a day in plastic feeding trays, and rations were adjusted according to apparent consumption. Water quality parameters were recorded in each pool during the entire experiment. Temperature and dissolved oxygen were measured twice daily at 6:00 a.m. and 1:00 p.m., while salinity and pH were recorded once daily. Organic matter and nutrients (orthophosphate, nitrite, nitrate, and ammonia) were recorded weekly. Shrimp growth was also monitored weekly. Survival, feed-conversion ratio, and final biomass were calculated at the end of the trial.
Martinez-Cordorva, Production parameters of Litopenaeus vannamei fed diets, Table 1
| Parameter | Control | Treatment 1 | Treatment 2 | Treatment 3 |
|---|---|---|---|---|
| Final weight (g) | 13.06 ± 1.00ab | 12.45 ± 0.76a | 14.89 ± 0.53b | 11.72 ± 1.41a |
| Weight gain (g) | 11.60 ± 1.28a | 11.13 ± 0.46a | 13.50 ± 0.26b | 10.58 ± 1.18a |
| Survival (%) | 62.19 ± 17.62a | 71.20 ± 21.13ab | 67.72 ± 5.66ab | 95.72 ± 9.90b |
| Consumed feed (kg) | 1,907 ± 130.8ab | 1,965 ± 90.4ab | 2,078 ± 26b | 1,738 ± 170a |
| Final biomass (kg) | 819.0 ± 282.3a | 877.4 ± 228.7a | 1,000.5 ± 49.7a | 1,117.3 ± 15.3a |
| FCR | 2.32 ± 0.54b | 2.23 ± 0.56b | 2.07 ± 0.14ab | 1.55 ± 0.14a |
Results
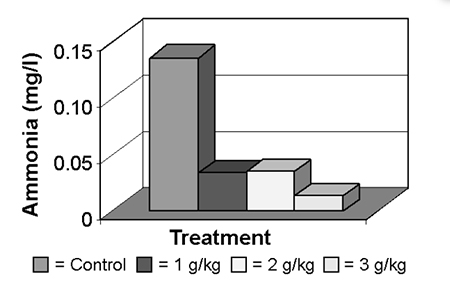
Ammonia concentration was significantly and consistently lower in the treatments that included the yucca extract, compared to the control. Treatment 3 recorded the lowest levels (Fig. 1). This confirmed the findings of other researchers who have worked with fish, crustaceans, and mammals using other products to minimize ammonia concentrations.
Some of the production parameters showed significant differences among treatments (Table 1). Weight gain was greater in treatment 2. However, survival and feed conversion were better in treatments 3 and 4. The low growth in treatment 3 was related to the high survival obtained in that treatment, mainly due to animal overcrowding.
Conclusion
The yucca extract effectively reduced ammonia concentration in the water column when given in feed to experimental shrimp. The most significant effect occurred with a concentration of 3.0 grams per kilogram of feed, but even the positive effect of 1 gram per kilogram the effect was evident. An analysis is still needed to determine what concentrations could produce the best economic results.
(Editor’s Note: This article was originally published in the June 2003 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Luis R. Martinez-Cordova, Ph.D.
Departamento de Investigaciones
Científicas y Tecnológicas
Universidad de Sonora
Rosales y Luis Encinas
P.O. Box 1819
Hermosillo, Sonora 83000 México[120,109,46,110,111,115,117,46,115,97,109,121,97,117,103,64,122,116,109,108]
-
Alfredo Campaña-Torres, M.S.
Departamento de Investigaciones
Científicas y Tecnológicas
Universidad de Sonora
Rosales y Luis Encinas
P. O. Box 1819
Hermosillo, Sonora 83000 México
Tagged With
Related Posts

Responsibility
A look at unit processes in RAS systems
The ability to maintain adequate oxygen levels can be a limiting factor in carrying capacities for RAS. The amount of oxygen required is largely dictated by the feed rate and length of time waste solids remain within the systems.

Innovation & Investment
A review of unit processes in RAS systems
Since un-ionized ammonia-nitrogen and nitrite-nitrogen are toxic to most finfish, controlling their concentrations in culture tanks is a primary objective in the design of recirculating aquaculture systems.

Responsibility
A review of water quality improvement products
Prof. Boyd examines products used by aquafarmers to improve water quality and conditions in their ponds and discusses their efficacy.

Responsibility
Accuracy of custom water analyses varies
The reliability of trace element analyses reported by custom laboratories cannot be checked by simple techniques, and results may not always be accurate. One should check the reliability of major ion analyses by determining the charge balance and comparing the measured total ion concentration with the total ion concentration estimated from conductivity.


